Heterogeneous catalytic strategies on the selective oxidation of glycerol into dihydroxyacetone and glyceric acid: a short review of recent research advances
Abstract
Glycerol is an important biomass platform molecule, produced as an abundant side-product in the biodiesel industry. The efficient conversion of glycerol through selective oxidation to value-added fine chemicals has been recognized as one of the most promising routes to meet the demand for escalating energy. However, glycerol oxidation usually involves the oxidation of the primary or secondary C–OH groups of glycerol, cascade oxidation of intermediates, and C–C oxidative cleavage of glycerol or its intermediates, resulting in multiple pathways and varied oxidative products. To achieve the selective conversion of glycerol to a single product is of great challenge. The development of efficient catalysts with precisely-designed active sites for the targeted activation of glycerol and the direct conversion to specific oxidative products is the key. In this review, we focus on the recent research advances of the selective oxidation of glycerol to the highly-desired C3 value-added chemicals including dihydroxyacetone and glyceric acid over heterogeneous catalysts and the synergistic catalytic mechanism. The strategies for the targeted and efficient activation of glycerol at the primary or secondary position and the direct conversion of the primary or secondary C–OH and C–H bonds are emphasized. At the end of the article, future challenges and development strategies on the selective oxidation of glycerol are also discussed.
Keywords
INTRODUCTION
Glycerol is an important biomass platform molecule, which was listed as one of the twelve most promising biomass platform compounds by the U.S. Department of Energy in 2004. It is produced as an abundant side-product in the biodiesel industry[1]. The production of every 1.0 kg of biodiesel leads to the formation of 0.1 kg of crude glycerol[2]. The amount of byproduct glycerol is expected to surpass 4.3 billion kg per year by 2026[3]. To address the significant global challenges in energy, environment, food, and other areas, biomass and platform molecules, recognized as clean and renewable resources, have been receiving increasing attention to meet the growing energy needs[4,5]. In this scenario, the efficient and high value-added conversion of side-product glycerol to fine chemicals and fuels is quite required more than ever before[6].
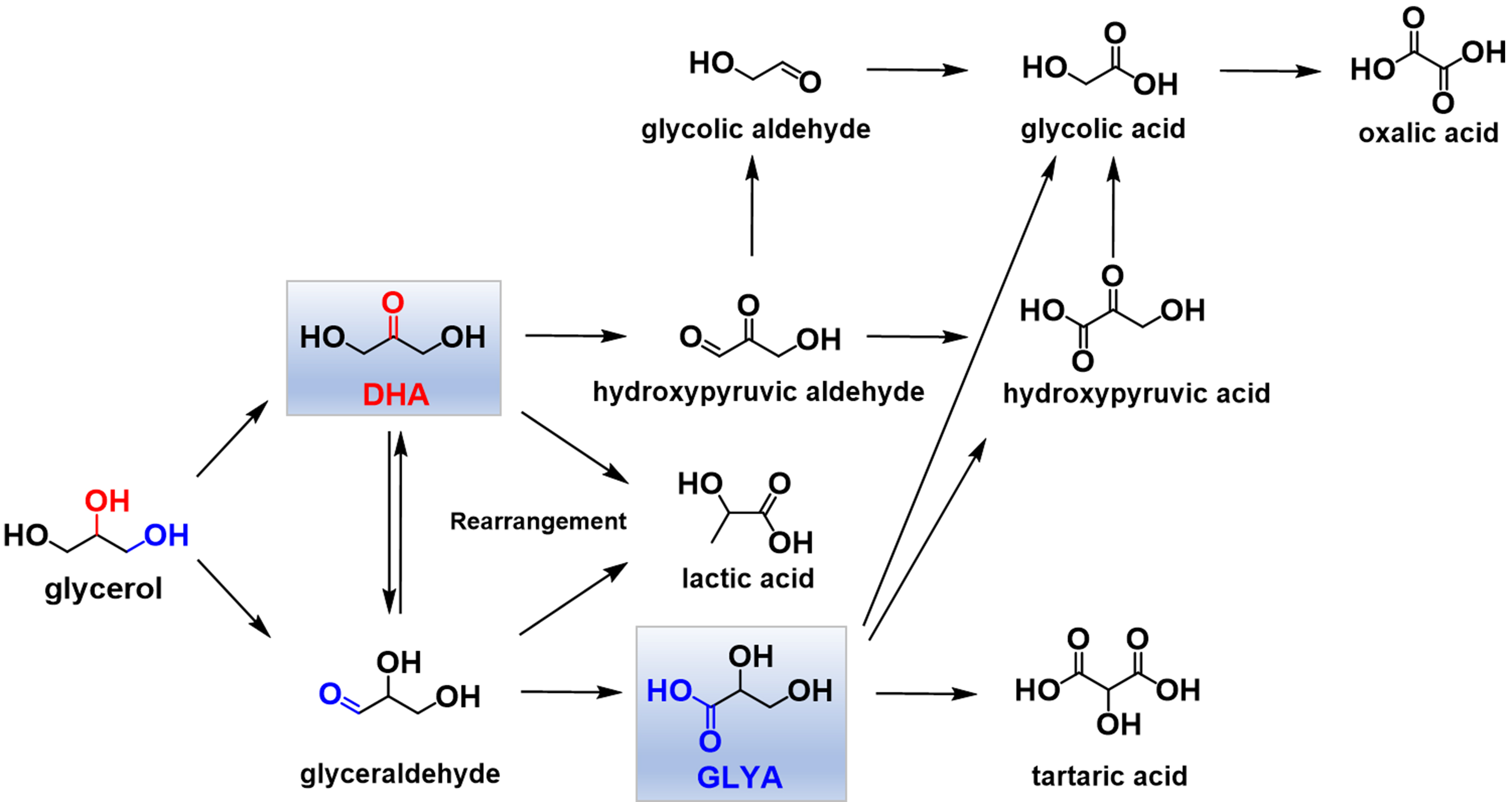
Glycerol could be converted into various highly-desired fine chemicals by the reactions containing oxidation[7-9], hydrogenolysis[10-12], dehydration[13-15], esterification[16-18], oxidative carbonylation[19-21], and so on. The oxidation pathway is a highly promising routes to achieve the glycerol-based value-added products including glyceraldehyde, dihydroxyacetone (DHA), glyceric acid (GLYA), glycolic acid, lactic acid, and more. Among these, DHA and GLYA command significant economic value, priced at $150 per kg and $7.5 per kg, respectively[22,23], which starkly contrasts with the relatively low price of glycerol at $0.1 per kg. They serve as crucial raw materials and intermediates, particularly in cosmetics, food, pharmaceuticals, and other chemical industries, holding broad market prospects[24,25].
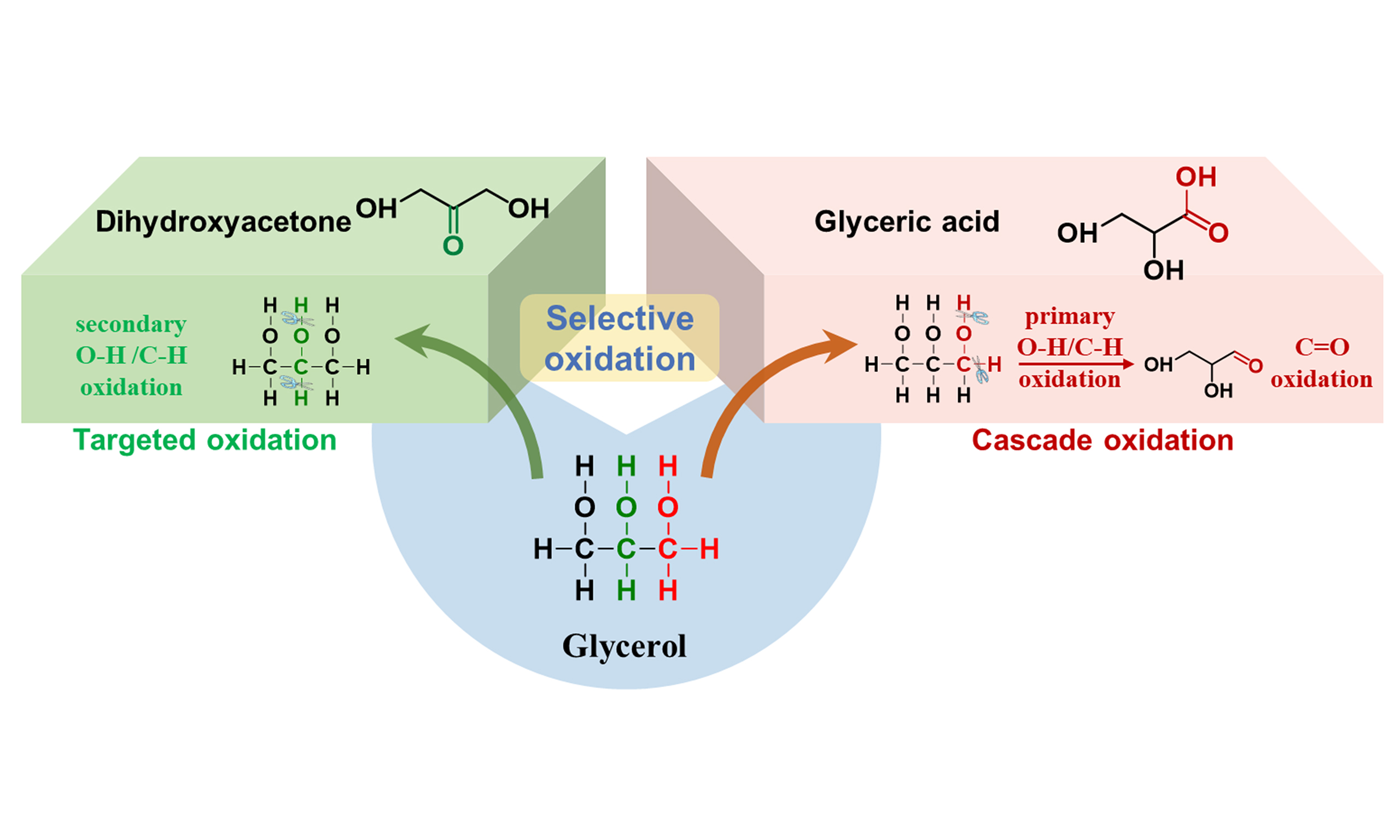
Glycerol is a typical polyol with three –OH groups, containing two primary –OH and one secondary –OH groups. Generally speaking, the catalytic oxidation of glycerol typically involves oxidation of the primary or secondary C–OH bonds of glycerol, cascade oxidation of the primary or secondary C–OH/C=O bonds of intermediates after dehydrogenation, and C–C oxidative cleavage, making glycerol oxidation proceed through multiple pathways and yield diverse oxidative C2 and C3 compounds [Figure 1]. In essence, firstly, the bond energies of C–H, C–C, and C–O bonds are comparable, making the scission of secondary O–H and C–H bonds prone to interference from deep oxidation and C–C bond scission[26]. Secondly, the thermodynamic energy for the primary and secondary C–H bond cleavage is very close, resulting in an inevitable emergence of the competitive oxidation[27]. Thirdly, the intermediates after dehydrogenation, such as aldehyde or ketone, are thermodynamically unstable and are more likely to occupy the active sites, thus undergoing the inevitable deep oxidation[28]. Achieving high selectivity to specific oxidation products requires the design of target reaction pathways that are bounded by thermodynamic constraints and fine-tuned by kinetic modulation. Deep oxidation side reactions, which are thermodynamically favored, are curbed by adjusting the properties of the support [acid-base characteristics or oxygen vacancy (VO) content] and optimizing reaction conditions (temperature, pressure, and solution pH). Meanwhile, the activation energy of the desired reaction pathway is reduced through the construction of interfacial active sites and multi-metallic synergistic sites, which are under kinetic control. This strategy enables the targeted activation of glycerol and its direct conversion to specific oxidation products.
Prior publications reviewed the recent progress on the thermo-[29,30], electro-[31-33], photo-[34-36], and biocatalytic glycerol oxidation[37]. While in the present work, we focus on a short review on thermo-catalytic glycerol oxidation including the catalyst design, the catalytic mechanism, and the reaction pathways for highly-desired C3 value-added chemicals including DHA and GLYA from glycerol transformations over heterogeneous catalysts. The strategies on the targeted and efficient activation of glycerol at the primary or secondary position and the direct conversion of the primary or secondary O–H and C–H bonds to the specific oxidative product are emphasized. This review is expected to provide insights into synergistic catalysis and offer guidance for designing efficient catalysts with multi-active sites.
TARGETED OXIDATION OF THE SECONDARY C-OH IN GLYCEROL TO DHA
DHA could be formed by the selective oxidation of the secondary C–OH groups in glycerol via oxidative dehydrogenation in the presence of molecular O2. The oxidation to DHA needs the dehydrogenation of both O–H and C–H bonds at the secondary position. However, it is not easy to give a preference to the activation of the secondary C–OH beyond the primary one. Moreover, the suppression of the C–C bond scission to C2 byproducts and even CO2 and the deep oxidation to the carboxylic acid compounds are full of challenges. Considerable reports on the development of heterogeneous catalytic strategies over well-designed multiple catalytic active sites to achieve the selective oxidation of glycerol to DHA under base-free conditions in the presence of molecular O2. Supported Au[38-48], AuM (M = Pd, Cu)[49,50], PtM (M = Bi, Sb)[51-56], PdM (M = Ag)[57] and PtSn[58] have demonstrated reasonable selectivity to DHA. Supported Au-based catalyst has been reported to be effective in the selective oxidation of secondary C–OH groups based on the density functional theory (DFT) calculation[38]. Monometallic Pt and Pd catalysts have been generally recognized to have the advantage in the oxidation of the primary C–OH over the secondary C–OH[27]. However, incorporating a second metal could effectively block the high-energy sites of Pt or Pd, enabling the adsorption of the primary C–OH groups on the second metal sites and being protective to be activated, thus improving the oxidation of the secondary C–OH groups to DHA[55].
Au-based catalysts
Reported Au-based catalysts for DHA production are generally supported on metal oxides (such as CuO[39] and ZnO[44-46,50]), and mixed metal oxides (such as MgO-Al2O3[40], Cu2O-MgO-Al2O3[41], ZnO-CuO[42,49], CuO-ZrO2[43], and CuZnOx[47]), along with layered double hydroxides (LDHs)[48]. The supports could offer basic sites and defects, and the strong interaction of the metal and supports could construct the interfacial active sites, facilitating the generation of DHA through the selective oxidation. To summarize, the selective oxidation of glycerol over Au-based catalysts typically involves the following steps: (1) the adsorption and activation of O2 on Au form O-O* species, which then reacts with H2O to produce *OOH and *OH species; (2) the secondary hydroxyl group of glycerol is selectively adsorbed to form R2CHO*, releasing H*, which combines with OH* to generate H2O; (3) the OOH* species attacks the β-carbon of glycerol, removing a hydrogen atom to yield DHA and H2O2[45,49]. Over supported Au, the synergistic catalysis of metal-support sites, metal-interface sites, and metal-metal sites has been embodied for the formation of DHA from glycerol.
Metal-support site synergy
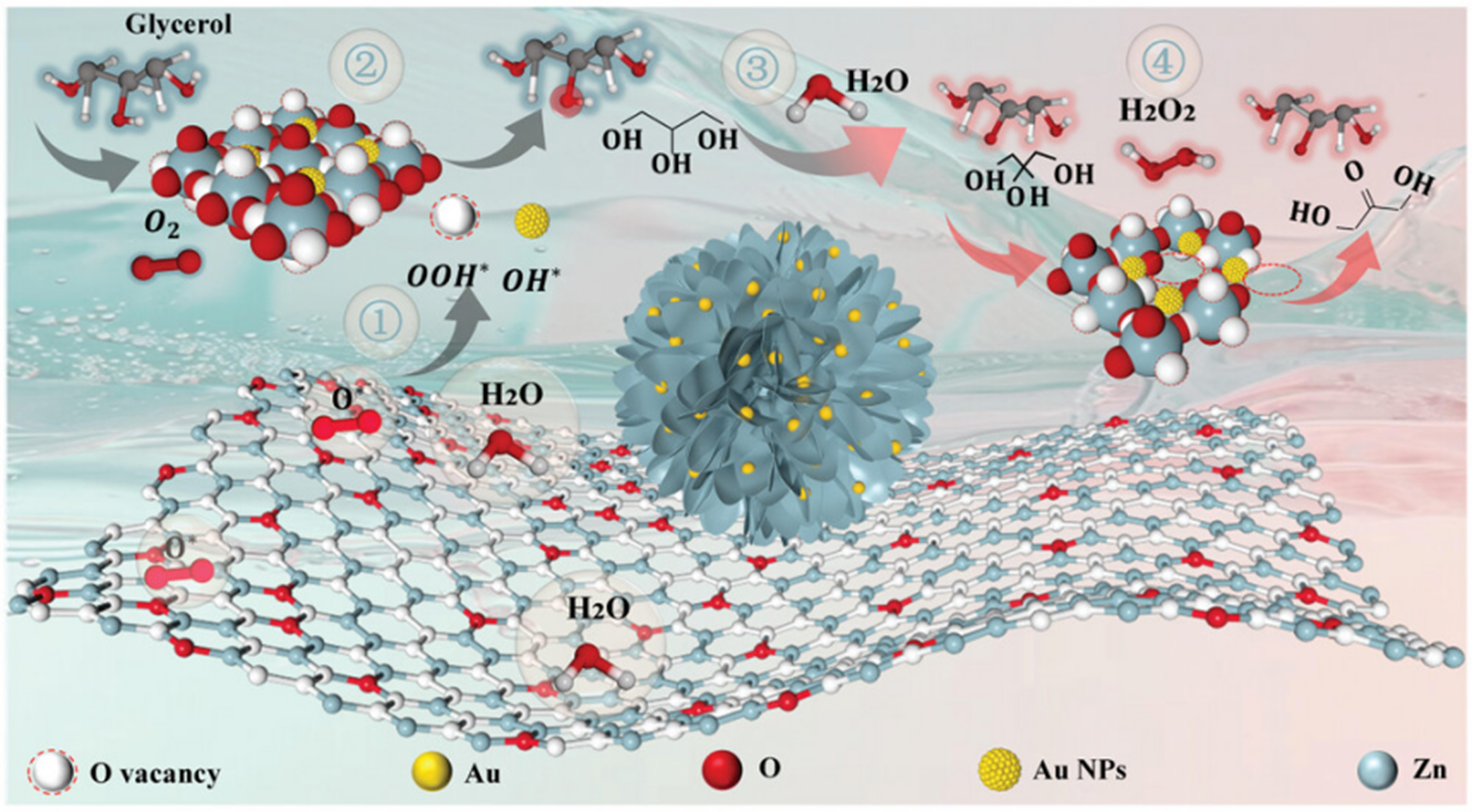
Early studies focused on the regulation on the Au nanoparticle size or/and the support natural properties of supported Au catalysts. Liu et al. reported for the first time that metal oxide supporting Au nanoparticles have the ability to catalyze the selective oxidation of glycerol to DHA in a selectivity of up to 80% at a low glycerol conversion of 20% in the presence of molecular O2 under base-free conditions[39]. The paper also discussed the effects of the metal oxide supports including Al2O3, TiO2, ZrO2, NiO, and CuO towards the catalytic performance. Further investigation by the same group[40] explored the influence of the acid-base properties of the support on the glycerol oxidation reaction over a series of Au/MgO-Al2O3 with varied molar ratios of Mg/Al. It revealed that the highest glycerol conversion and DHA selectivity were achieved over the most acidic with the least basic Au/MgO-Al2O3 with a Mg/Al ratio of 0.1, achieving a glycerol conversion of 16.4% and a selectivity of 74.1% to DHA. These findings underscore the critical role of the acid-base characteristics of supports in determining the selective oxidation products of glycerol. Then, Yin et al. covered that an enhanced catalytic performance in a glycerol conversion of 53% and a selectivity of 72% to DHA were obtained through a synergy between Cu+ species and the support basic sites over Au nanoparticles supported on Cu2O-MgO-Al2O3 mixed oxides[41]. Wang et al. loaded Au nanoparticles onto a series of mineral-derived CuO-ZnO mixed oxides[42]. It is found that Au/CuO-ZnO prepared from rosasite precursor, which possessed the Au0 content of 78.9%, the Au particle size of 2.24 nm, and the basicity density of 0.0420 mmol/gcat, achieved a glycerol conversion of 71.6% and a DHA selectivity of 93.2%. Wang et al. tailored the particle size of Au and the basicity in CuO-ZrO2 supporting Au nanoparticles by modulating the calcination temperature[43]. It is found that Au/CuO-ZrO2 possessing an Au particle size of 2.1 nm with a higher Au0 site content of up to 95% and the surface basicity of 0.043 mmol/gcat exhibited a glycerol conversion of 73% and a selectivity of up to 95% to DHA. Xiong et al. prepared Au nanoparticles loaded on a flower-shaped ZnO (Au/ZnO-NF), which had a large specific surface area of 42 m2·g-1[44]. Au/ZnO-NF displayed the Au particle size as small as 1.73 ± 0.70 nm and a high VO content of 40.4%, facilitating the adsorption and the activation of the secondary O–H groups in glycerol [Figure 2]. Consequently, Au/ZnO-NF achieved a glycerol conversion of 92.9% and a selectivity of 69.5% to DHA, along with remarkable recycling stability.
Figure 2. Catalytic mechanism of Au/ZnO-NF for the secondary O–H group oxidation[44]. Copyright 2025, Wiley-VCH GmbH.
Metal-interface site synergy
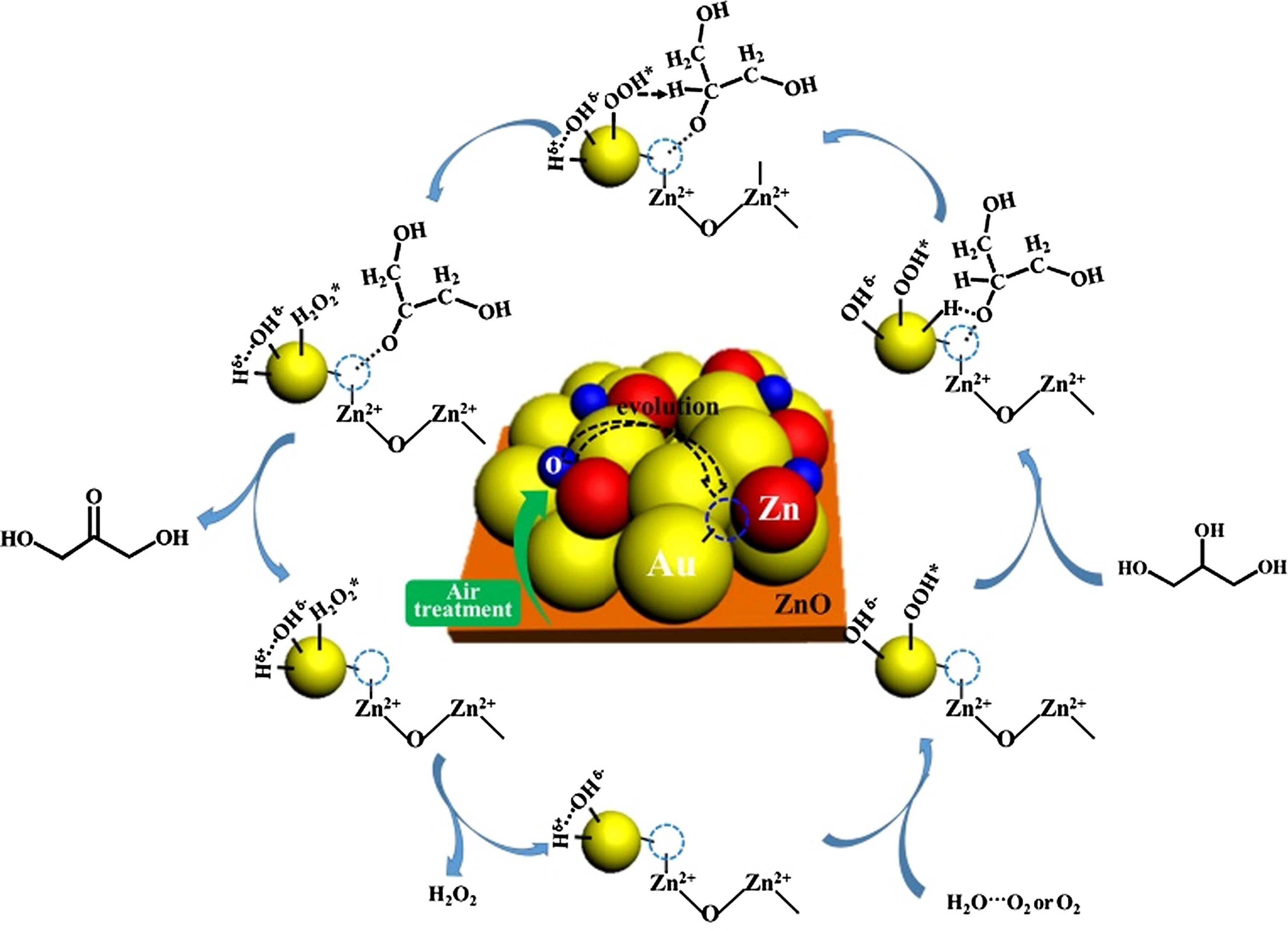
Recently, Pan et al. fabricated Au/ZnO in a strong Au-support interaction and identified the Au-O-Zn interfacial species with incorporated VO as the active sites for the specific oxidation of secondary C–OH groups [Figure 3][45]. The glycerol conversion was determined as 86.4% and the selectivity to DHA as 62.1%. Later, the same group[46] further regulated the VO content in Au/ZnO with the Au-O-Zn interfacial structure, clarifying that the more the surface VO content (up to 45.4%), the more it is favorable for the targeted oxidation of the secondary C–OH groups. Yuan et al. prepared Au/CuZnOx@bZ composites with varied interfacial binding modes by modulating the assembly process of bio-templated porous ZSM-5 zeolite platform (bZ) and Au/CuZnOx[47]. Among them, Au/CuZnOx@bZ prepared by physical milling mode presented the highest surface Au+ content of 33.7%, illustrating the most Au-O-T (Zn/Cu/Si) interfaces. This increase on the interfacial sites significantly enhanced the catalytic performance in the glycerol oxidation, achieving a glycerol conversion of 93% with a specific selectivity of up to 86% to DHA. These findings underscore the crucial role of interfacial engineering in improving the catalytic efficiency.
Figure 3. Proposed catalytic mechanism for the selective oxidation of glycerol to DHA over Au–O–Zn interfacial structure in Au/ZnO[45]. Copyright 2019, Elsevier. DHA: Dihydroxyacetone.
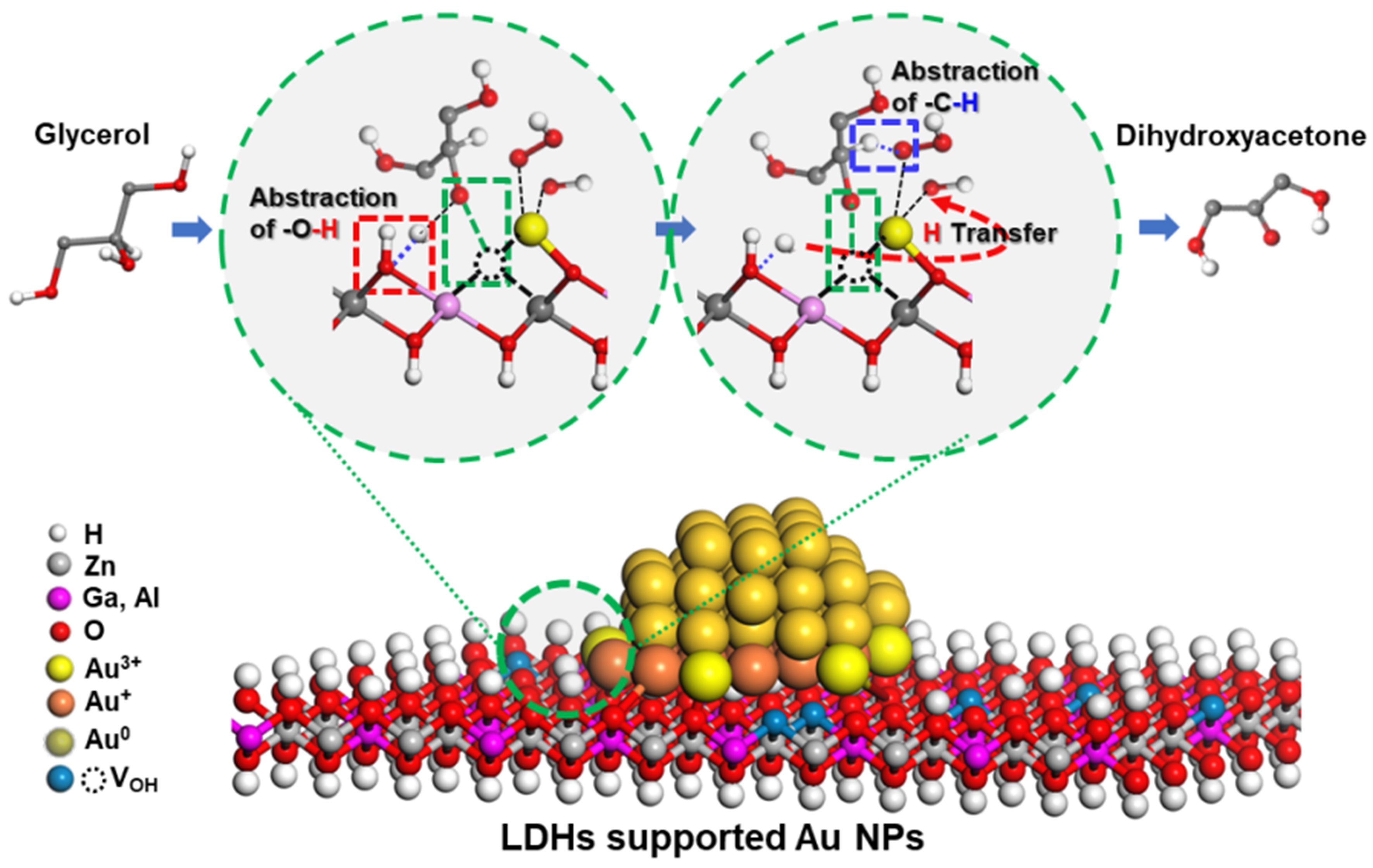
Our research group[48] prepared Zn2Fe-, Co2Al-, Zn2Al-, Zn2Ga-, and Mg2Al-LDHs supporting Au nanoparticles, which display tailored surface basic density and the hydroxyl vacancies (VOH), in the selective oxidation of glycerol to DHA. It is found that LDHs could provide a strong interaction between Au nanoparticles and surface hydroxyl groups to establish a large number of the interfacial MII-O-Aun+ species. The cooperation of the surface basic –OH groups and interfacial MII-O-Au+ species to generate a synergistic catalysis on the activation of both the secondary O–H bonds and the secondary C–H bonds has been clarified [Figure 4]. More importantly, over Zn-based LDHs, an extra synergistic effect provided by the surface VOH species and the interfacial ZnII-O-Au3+ sites has been verified, resulting in an enhancement of the glycerol conversion and the selectivity to DHA. Au/Zn2Ga-LDHs afforded a selectivity of 63.8% ± 0.2% to DHA at a glycerol conversion of 72.9% ± 0.2%.
Figure 4. Proposed catalytic mechanism for the selective oxidation of glycerol to DHA by the synergistic catalysis of the surface basic sites and the interfacial MII-O-Au+ sites in LDHs supported Au nanoparticles[48]. Copyright 2020, American Chemical Society. DHA: Dihydroxyacetone; LDHs: layered double hydroxides.
Metal-metal site synergy
Au-based bimetallic catalysts have also been reported to improve the electronic structure of Au and/or the surface defect structure. Zhao et al. prepared ZnO-CuO supporting AuPd alloy nanoparticles, achieving a DHA yield of up to 65.3% under base-free conditions[49]. It is indicated that the improved catalytic properties of the AuPd/ZnO-CuO were mainly attributed to the abundant surface VO species. Zhao et al. reported ZnO supporting AuCu alloy nanoparticles with rich interfacial Au-Cu-ZnO active sites towards the selective oxidation of glycerol to DHA[50]. The increased electron transfer capability from Au and Cu was verified to increase the content of Au+ species, thus resulting in a high catalytic activity [turnover frequency (TOF): 402.5 h-1]. The forming interfacial VO species induced the activation and adsorption of the secondary O–H bond in glycerol on the interfacial Au-Cu-ZnO sites, improving the selectivity of 83.4% to DHA. Moreover, the in situ Fourier transform infrared (FTIR) spectra and the DFT calculation demonstrated that Au-Cu-ZnO interfacial sites enhanced the participation of *OH and VO species, which activated the secondary O–H bonds and the C–H bonds, respectively.
Au-based catalysts show specific selectivity for the oxidation of the secondary C–OH in glycerol in the presence of O2. Optimized catalytic performance has been achieved by modulating the catalyst structures involving smaller Au particle size (preferably 2-5 nm), moderate alkalinity, more defects (VO and VOH), and more interfacial active sites (Au-O-MII), respectively. However, further elucidation on the catalytic mechanisms on these structural factors towards the (e.g., direct oxidation vs. glycerol aldehyde isomerization) towards the activity and selectivity are still needed, possibly through in situ advanced characterization methods.
Pt and Pd-based catalysts
Supported PtBi[51-55], PtSb[56], PtSn[58], and PdAg[57] have been reported to achieve a dominant selectivity to DHA in the glycerol oxidation. Currently, two possible catalytic mechanisms have been reported: (1) spatial site-blocking effect: Introducing a second metal to block the high-energy sites on Pt/Pd, thereby enabling Pt/Pd to preferentially adsorb the activated secondary hydroxyl group of glycerol[51-57]; (2) electronic effect: Regulating Pt to an electron-deficient state, which facilitates the isomerization of glycerol aldehyde to DHA[58].
Spatial site-blocking effect
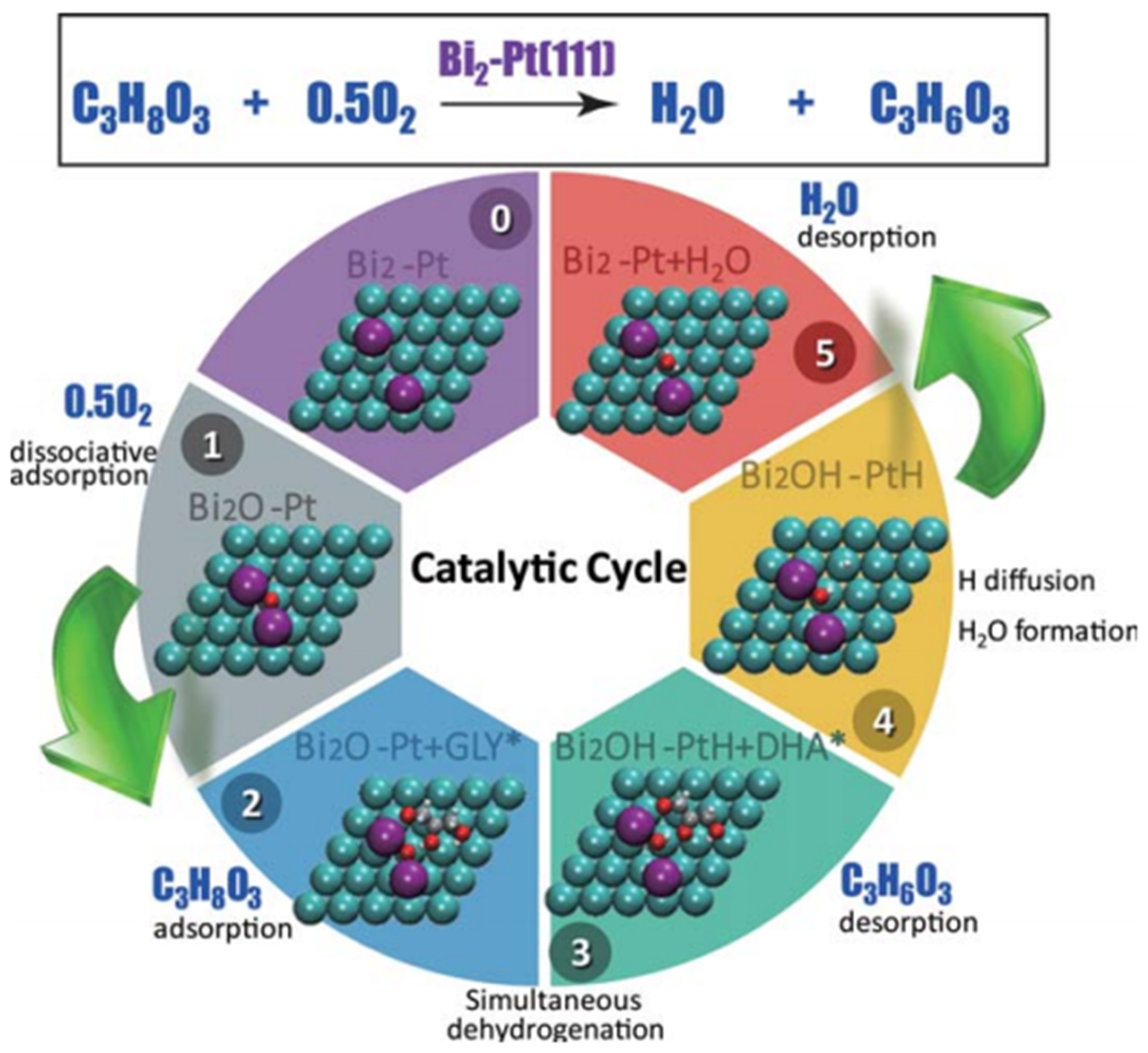
The structure of Bi-promoted Pt and the role of Bi additive in supported PtBi have been investigated intensively by several groups[51-55]. Ning et al. introduced soluble Bi3+ species into the Pt/N-carbon nanotube (CNT) catalyzed glycerol oxidation system, and DHA was detected as the dominant product instead of glyceraldehyde without Bi addition[51]. They concluded the Bi3+ additive covered the step sites of Pt nanoparticles in high energy, and then the terrace sites in mediate energy, forming Bi-Pt species on the terrace, which significantly improved the catalytic performance in the glycerol oxidation by the geometric blocking effect. Xiao et al. prepared Pt-Bi/MCM-41, Pt/MCM-41, and Pt/Bi doped MCM-41, among which Pt-Bi/MCM-41 was detected to possess the highest DHA yield [Figure 5][52]. Combined with the experimental results and the DFT calculation, they proposed a possible heterogeneous catalytic cycles: (1) O2 is firstly adsorbed on a Bi site in dissociative mode on Bi2-Pt(111) surface forming Bi2O-Pt(111); (2) Glycerol was then adsorbed in the secondary position at the interface between Bi2O and Pt(111), establishing a Bi2O-Pt + GLY* complex; (3) The dehydrogenation of glycerol to DHA (Bi2OH + PtH + DHA*) takes place followed by the desorption of DHA (Bi2OH + PtH), and the catalytic cycle finishes with the final formation and the release of H2O as well as the recovery of Bi2-Pt. More recently, Feng et al. prepared Pt-Bi/SBA-15 achieving a glycerol conversion of 40.9% and a selectivity of 65.1% to DHA at 303 K under air in a base-free solution[53]. They concluded that three O–H groups of glycerol were all captured by Bi species, and the secondary O–H simultaneously interacted with Pt, leading to the selective oxidation of glycerol to DHA. Tang et al. reported the surface reconstruction between single-atom Bi-N-C and Pt nanoparticles[54]. The strong binding between Pt and N facilitated a thermodynamic exchange of Pt and Bi atoms, forming stable Pt-Bi alloy nanoparticles (< 2 nm). The unimpeded Bi–N bond driven by ring-breaking kinetics, ensured the uniform distribution of Pt-Bi clusters. A glycerol conversion of 88.0% with a TOF value of 224.4 h-1 and a DHA selectivity of 64.9% was achieved over 5Pt/Bi50NC-900 (H2). Yu et al. compared the catalytic performance for the glycerol oxidation over Pt loaded on Bi2O3-CNTs prepared by the atomic layer deposition (ALD) and the impregnation method[55]. It is found that Pt/Bi2O3-CNTs by the ALD method exhibited an abundant Pt-Bi2O3 interface, which created the fine-tuning on the electronic and geometrical structure of Pt. The positively-charged Pt species at the interface facilitated glycerol dehydrogenation and enhanced glycerol adsorption, thereby suppressing over-oxidation reactions. As a result, Pt/Bi2O3-CNTs by ALD achieved a glycerol conversion of 85.3% and a selectivity of 56.7% to DHA.
Figure 5. Proposed catalytic mechanism over Pt-Bi in the selective oxidation of glycerol to DHA[52]. Copyright 2016, American Institute of Chemical Engineers. DHA: Dihydroxyacetone.
In addition to PtBi, Duan et al. found that Sb, an element of the same main group as Bi, could also generate the oriented conversion of glycerol to DHA[56]. Theoretical calculations and experimental results revealed that the presence of Sb species was in the form of SbOx on PtSb. Notably, the secondary O–H group of glycerol had the longest O–H bond lengths, indicating that activation of the secondary O–H group on the surface of SbO-Pt(111) was more favorable in comparison with the Pt(111) surface without the modification of Sb.
Besides Pt-based bimetallic catalysts, Hirasawa et al. prepared PdAg/C, which displayed a DHA selectivity of up to 85% at a glycerol conversion of 52%[57]. It is found that the introduction of Ag obviously enhanced the sorption of glycerol on the surface, where the primary O–H bonds of glycerol were adsorbed on Ag, while the adjacent secondary O–H bonds on Pd, leading to a high DHA selectivity.
Electronic effect
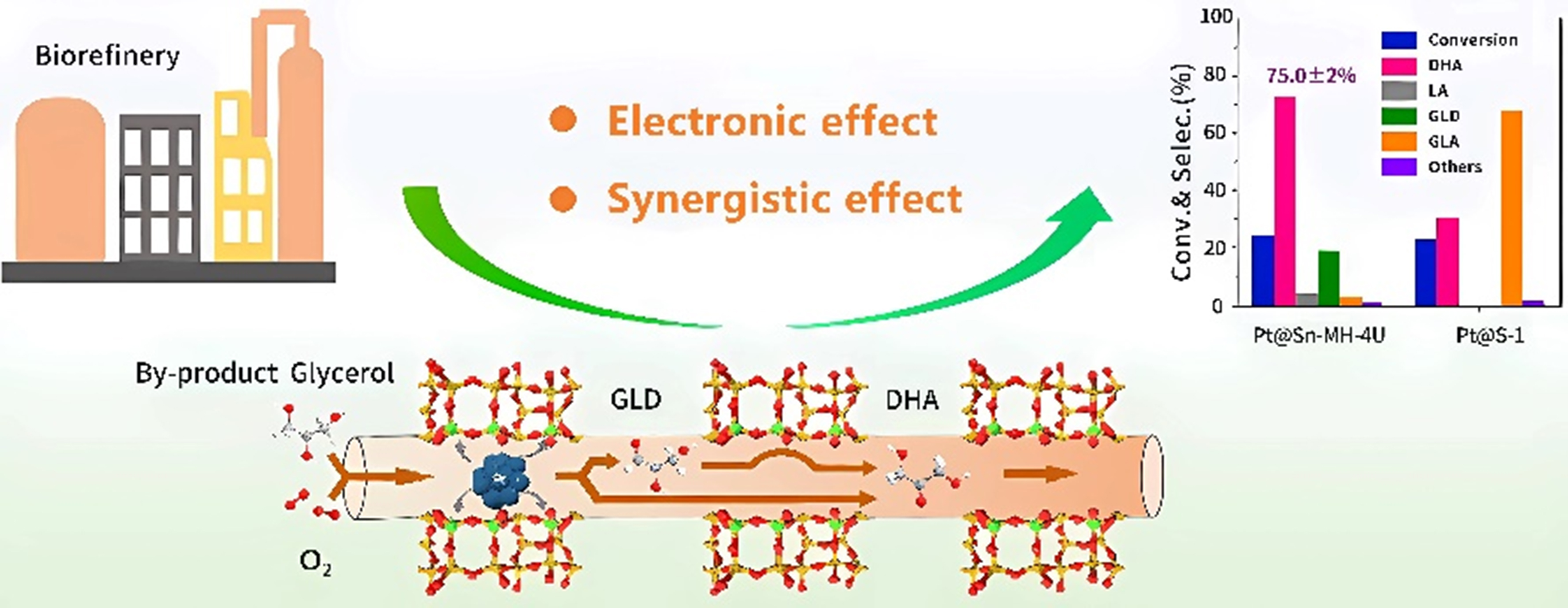
Moreover, Wang et al. synthesized a series of Pt@Sn-MFI in core-shell structure, where the Pt nanoparticles were encapsulated within the Sn-MFI framework, and the interaction between Pt and Sn induced an electron-deficient state in Pt[58]. Pt@Sn-MFI was proposed to achieve a two-step tandem oxidative conversion of glycerol through the synergistic interaction between Pt nanoparticles and the Sn-MFI shell: (1) Pt nanoparticles were preferentially activated at the glycerol adsorption site to selectively produce glyceraldehyde as intermediate; (2) Sn species modulated Pt into an electron-deficient state, which inhibited the oxidation of glyceraldehyde to GLYA; (3) Ultimately, glyceraldehyde was further converted to DHA on the Sn-MFI shell [Figure 6]. As a result, Pt@Sn-MFI-80 achieved a DHA selectivity of 74.8% and a glycerol conversion rate of 43.5%.
Figure 6. Catalytic mechanism for the highly selective oxidation of glycerol to DHA over Pt@Sn-MFI[58]. Copyright 2024, American Chemical Society. DHA: Dihydroxyacetone.
To sum up, Table 1 summarized the typical catalysts discussed above with their catalytic performances in the selective oxidation of glycerol to DHA under specific conditions.
Typical catalysts used for the selective oxidation of glycerol to DHA
| Catalysts | Reaction conditions | Conv. (%) or TOF (h-1) | DHA selectivity (%) | Ref. |
| 2Au/CuO | 0.1 M glycerol, glycerol/Au (mol/mol) = 1,000, 80 °C, O2 10 bar, 900 rpm, 2 h | 20.6 | 82.5 | [39] |
| Au/MgO-Al2O3 | 0.1 M glycerol, glycerol/Au (mol/mol) = 315, 80 °C, O2 10 bar, 900 rpm, 2 h | 16.4 | 74.1 | [40] |
| Au/CuMgAl-HTs | 0.3 M glycerol, glycerol/Au (mol/mol) = 1,000, 60 °C, O2 3 bar, 400 rpm, 6 h | 53.0 | 72.0 | [41] |
| Au/CuO0.9ZnO0.1 | 0.1 M glycerol, glycerol/Au (mol/mol) = 50, 50 °C, O2 0.2 bar, 700 rpm, 4 h | 71.6 | 93.2 | [42] |
| Au/Cu0.95Zr0.05 | 0.1 M glycerol, glycerol/Au (mol/mol) = 50, 50 °C, O2 0.2 bar, 700 rpm, 4 h | 72.8 | 96.0 | [43] |
| Au/ZnO-NF | 0.1 M glycerol, glycerol/Au (mol/mol) = 100, 100 °C, O2 1 bar, 500 rpm, 1 h | 92.9 | 69.5 | [44] |
| Au/ZnO-DP-N-Air | 0.1 M glycerol, glycerol/Au (mol/mol) = 100, 80 °C, O2 1 bar, 800 rpm, 5 h | 86.3 | 59.5 | [45] |
| Au/ZnO-U | 0.1 M glycerol, glycerol/Au (mol/mol) = 100, 80 °C, O2 1 bar, 900 rpm, 2 h | 75.9 | 74.2 | [46] |
| Au/CuZnOx@bZ | 0.1 M glycerol, glycerol/Au (mol/mol) = 100, 80 °C, O2 1 bar, 500 rpm, 2 h | 93.0 | 86.0 | [47] |
| 0.98 wt%Au/ZnGa-LDHs | 0.1 M glycerol, glycerol/Au (mol/mol) = 200, 60 °C, O2 5 bar, 700 rpm, 4 h | 73.1 | 63.6 | [48] |
| AuPd/ZnO-CuO | 0.05 M glycerol, glycerol/Au (mol/mol) = 100, 80 °C, O2 1 bar, 900 rpm, 2 h | 76.1 | 86.0 | [49] |
| AuCu-ZnO | 0.1 M glycerol, glycerol/Au (mol/mol) = 100, 60 °C, O2 15 bar, 1000 rpm, 5 h | 90.6 | 83.4 | [50] |
| PtBi5/NCNT | 0.1 M glycerol, glycerol/Pt (mol/mol) = 195, 60 °C, O2 150 Ncm3/min, 600 rpm, 6 h | 30.4 | 55.5 | [51] |
| PtSb1/NCNT | 0.1 M glycerol, glycerol/Pt (mol/mol) = 195, 60 °C, O2 150 Ncm3/min, 600 rpm, 6 h | 50.9 | 38.1 | [51] |
| Pt-Bi/MCM-41 | 1.0 M glycerol, glycerol/Pt (mol/mol) = 228, 75 °C, O2 400 Ncm3/min, 2.8 h | 2.8* | 64.3 | [52] |
| 3Pt-0.3Bi/SBA-15 | 0.2 M glycerol, glycerol/Pt (mol/mol) = 65, 30 °C, Air 1 atm, 15 h | 40.9 | 65.1 | [53] |
| 5Pt/Bi50NC-900(H2) | 0.1 M glycerol, glycerol/Pt (mol/mol) = 670, 60 °C, O2 150 Ncm3/min, 1,200 rpm, 6 h | 88.0 | 64.9 | [54] |
| Pt5C/Bi2O3-CNTs | 0.04 M glycerol, glycerol/Pt (mol/mol) = 850, 60 °C, O2 150 mL/min, 1,100 rpm, 6 h | 85.3 | 56.7 | [55] |
| Pt-Sb/CNTs | 0.1 M glycerol, glycerol/Pt (mol/mol) = 890, 60 °C, O2 150 cm3/min, 500 rpm, 2 h | 70.0 | 74.0 | [56] |
| Pd-Ag/C | 0.2 M glycerol, glycerol/Pd (mol/mol) = 1,150, 80 °C, O2 1 bar, 1,000 rpm, 4 h | 20.0 | 82.2 | [57] |
| Pt@Sn-MFI-80 | 0.1 M glycerol, glycerol/Pt (mol/mol) = 670, 60 °C, Air 40 mL/min, WHSV = 0.055 h-1 | 43.5 | 74.8 | [58] |
| Pt/Bi@NC | 0.1 M glycerol, glycerol/Pt (mol/mol) = 76, 30 °C, Air 1 atm, 15 h | 86.8 | 87.0 | [59] |
| AuPd/CNT | 0.1 M glycerol, glycerol/Au (mol/mol) = 1,000, 110 °C, O2 10 bar, 2 h | 87.0 | 70.1 | [60] |
| Au/CuO@bio-ZSM-5 | 0.1 M glycerol, glycerol/Au (mol/mol) = 100, 80 °C, O2 10 bar, 2 h | 97.3 | 92.2 | [61] |
(1) Au-based catalysts present specific activation of the secondary O–H groups in glycerol, achieving a DHA selectivity of 70%-90% at a moderate glycerol conversion over Au nanoparticles (2-5 nm) supported on solid base. Generally speaking, in the oxidation reaction of glycerol, the oxidation product, i.e., aldehyde, ketone, or acid, is more readily adsorbed on Au active sites than glycerol, leading to the coverage of the active sites and thus the inhibition of glycerol adsorption and activation. As a result, the conversion is relatively low with a high selectivity of products derived from the secondary O–H oxidation.
(2) While Pt or Pd-based catalysts require a second metal (Bi/Sn/Sb/Ag) to provide the spatial site-blocking or/and the electronic modulation of Pt or Pd active sites, suppressing the thermodynamically favorable adsorption of the primary O–H groups and achieving the dominant adsorption of the secondary O–H groups. A glycerol conversion of > 80% with a moderate DHA selectivity over supported bimetallic Pt or Pd (2-3 nm), suffering from oxidation at the primary position and overoxidation. In essence, the oxidation efficiency is particularly high over Pt or Pd active sites, where the targeted adsorption and activation at the secondary position is insufficient.
To break through the “seesaw” relationship between the glycerol conversion and the DHA selectivity, the achievement of simultaneously high value of glycerol conversion and the DHA selectivity is still a great challenge.
CASCADE OXIDATION OF THE PRIMARY C–OH OF GLYCEROL TO GLYA
The selective oxidation of glycerol to GLYA requires the oxidative dehydrogenation of both the primary
Pt group metal-based catalysts, such as Pt and Pd, have been generally recognized to have the advantage in the oxidation of the primary C–OH over the secondary C–OH in the glycerol selective oxidation[27]. At present, Pt/Pd nanoparticles supported on carbon[64,65,69,78,80], metal oxides[66,70,72,79,81], mesoporous materials[71,73,77] and their composite materials (Pt/MnO2/CNT[74], Pt-CeO2/CNT[75], Pt/MgO/SBA-15[76]) are predominantly employed as the catalyst for the GLYA production from glycerol. The selective oxidation of glycerol over Pt-based catalysts typically involves the following steps: (1) O2 is adsorbed and activated on the Pt surface to form O*, which then reacts with H2O to produce OOH* and OH*; (2) The formed OH* species extract protons from the primary C–OH of glycerol and the OOH* species attack the α-carbon of glycerol, removing a hydrogen atom to yield glyceraldehyde as intermediates; (3) Glyceraldehyde is further oxidized in terms of the O–H insertion and the dehydrogenation of C–H bonds producing GLYA. The synergistic catalytic effects of both metal-support and metal–metal sites are highlighted in the selective oxidation of glycerol to GLYA over supported Pt and Pd catalysts.
Metal-support site synergy
Early work[64] focused on the dehydrogenation of primary C–H bonds of glycerol to improve the catalytic activity over the supported Pt nanoparticles in a base-free aqueous solution. Efforts to optimize glycerol catalytic oxidation included adjusting the particle size, morphology, and exposed crystal facets of the Pt nanoparticles. Liang et al. investigated the particle size effect of supported monometallic Pt towards the selective oxidation of glycerol to GLYA[65]. They discovered that glycerol conversion was consistently stable at 50% when the particle size was in the range of 1.2-6.3 nm. With the increasing Pt particle size, the glycerol conversion decreased significantly and declined to only 20% at 11.6 nm. Li et al. investigated the influence of the shape of Pt nanoparticles supported on SiO2 on the glycerol oxidation[66]. It is found that the octahedral Pt nanoparticles exhibited higher activity [turnover number (TON) = 148] compared to the tetrahedral Pt nanoparticles (TON = 58), with a comparable selectivity of GLYA (70%). Pt (111) crystal verified that the glycerol adsorption occurred at the primary position, which favored the formation of glyceraldehyde as intermediate in the dehydrogenation process. Yan et al. disclosed the catalytic mechanism for the glycerol oxidation on the Pt (111) surface[67]. The co-dissociation of oxygen and water polarized the Pt surface and generated active sites. Then O–H and C–H bonds at the primary position were broken to produce glyceraldehyde, which was further oxidized with adsorbed *OH to produce GLYA. DFT calculations[68] showed that the reaction pathway for the generation of *COOH upon further oxidation to GLYA on the Pt (111) surface with glyceraldehyde had the lowest activation energy (0.18 eV), which was lower than the retention of C=O (0.28 eV) and the removal of C=O (0.26 eV). However, due to the thermodynamic instability, GLYA tended to break the C–C bond when it was peroxidized leading to the generation of C2 byproducts and even CO2.
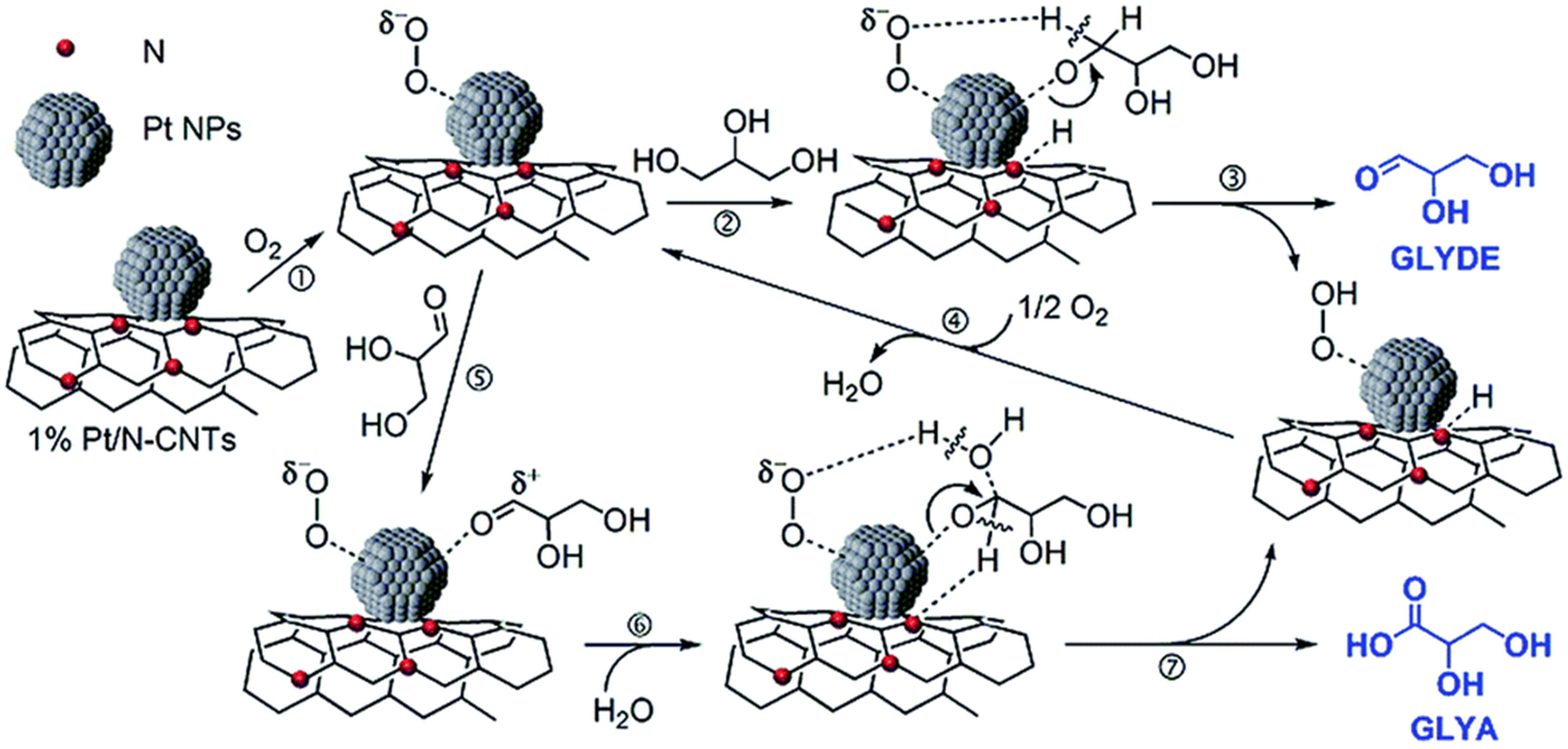
Further reports on the modulation of the electronic or the coordination structure of Pt nanoparticles through the nature of the supports, which could promote the metal-support interactions and construct more active sites to improve the catalytic performance. Chen et al. prepared a series of Pt/N-CNTs with tailored nitrogen contents[69]. They found that the incorporation of N atoms not only enhanced the surface basicity but also facilitated the activation of O2 via the electron transfer from N to Pt. The activated O2 and the primary O–H groups of glycerol were simultaneously adsorbed on the Pt surface, where they underwent dehydrogenation to form glyceraldehyde [Figure 7]. Subsequently, the oxygen species adsorbed on Pt reacted with water to produce GLYA. The catalytic pathway achieved a selectivity of 55.6% to GLYA at a glycerol conversion of 76.1%. Tan et al. prepared Pt nanoparticles supported on the carbon support functionalized with surface oxygen functional groups (SOFGs) in a content of 6.65%[70]. The improved hydrophilicity and basicity of the modified carbon surface promoted the Pt dispersion and consequently enhanced the glycerol adsorption and activation. Accordingly, the glycerol conversion was boosted from 9.75% to 67.6% and the GLYA selectivity was increased from 49.8% to 78.3% by comparison to the unmodified carbon supporting Pt. Zhang et al. developed Pt nanoparticles supported on LDHs through calcination-recovery method (re-MgxAl1-LDH-Pt) with regulated metal-support interaction and the basicity density by tailoring the molar ratio of Mg/Al in LDHs[71]. The formed interfacial Ptδ+ species facilitated the cleavage of the primary C–H bonds, synergistic with the surface basic sites promoting the oxidation of primary O–H bonds to produce GLYA. They revealed a volcano curve between the GLYA selectivity and the basicity density of the support. The highest GLYA selectivity of 66.9% at a glycerol conversion of 87.6% was obtained over re-Mg6Al1-LDH-Pt with a surface basicity density of 0.23 mmol/g and a Ptδ+/(Pt0 + Ptδ+) molar ratio of 0.32 at the Mg/Al molar ratio of 6.
Figure 7. Proposed reaction pathway for glycerol oxidation to GLYA catalyzed by Pt/N-CNTs[69]. Copyright 2015, The Royal Society of Chemistry. GLYA: Glyceric acid.
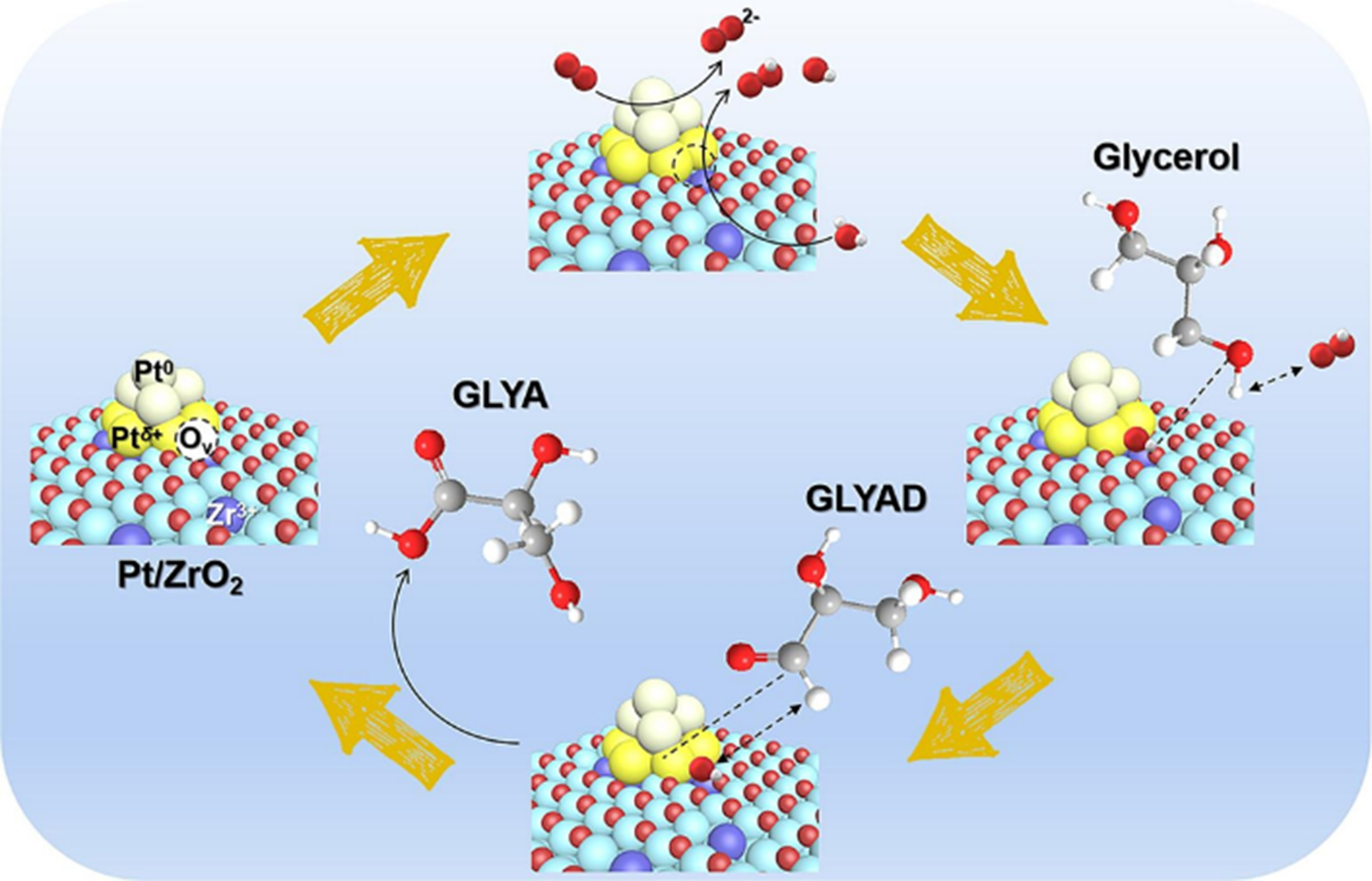
Recently, Ren et al. prepared Pt nanoparticles supported on monoclinic ZrO2 (Pt/ZrO2), featuring a unique interfacial structure characterized by Ptδ+-Ov-Zr3+ species[72]. A synergistic catalysis over Ptδ+-Ov-Zr3+ species was elucidated: (1) O2 molecules were activated and adsorbed on Pt nanoparticles to produce reactive oxygen species (O22-). VO sites in Ptδ+-Ov-Zr3+ species activated O22- species and H2O to generate *OOH and
Figure 8. Proposed catalytic mechanism of the glycerol selective oxidation to GLYA over Pt/ZrO2[72]. Copyright 2023, Elsevier B.V. GLYA: Glyceric acid.
Metal-metal site synergy
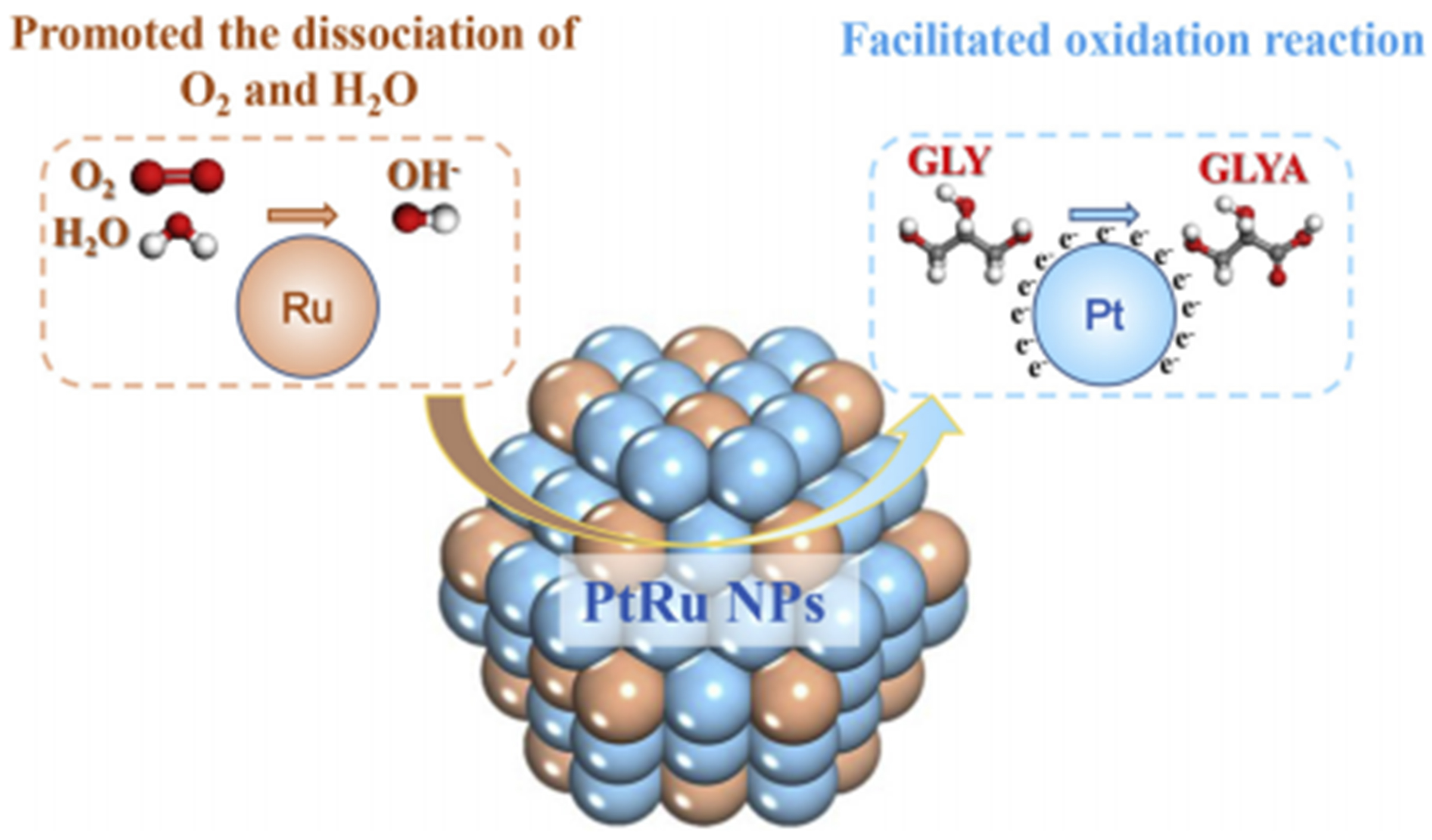
Another feasible route to improve the electronic structure and the local environment surrounding Pt is the incorporation of a second metal to construct bimetallic species in the selective oxidation of glycerol to GLYA. Liu et al. prepared AuPt bimetallic sites supported on MgO/SBA-15[76]. The incorporation of Au significantly reduced the cleavage energy for the primary C–H bonds in glycerol from 8.51 kcal/mol on Pt (111) to 4.64 kcal/mol on AuPt (111). A selectivity of 65.9% to GLYA at a glycerol conversion of 63.2% was achieved over supported AuPt. Moreover, the modification also enhanced the adsorption of glyceraldehyde on the AuPt surface, facilitating the tandem oxidation to produce GLYA. Yan et al. prepared MCM-41 supporting bimetallic PtRu, in which the alloying effect significantly reduced the size of PtRu nanoparticles to 1.9 nm[77]. The strong interaction between Pt and Ru coupled with the enhanced electron transfer was found to promote the direct dissociation of O2 and water into *OH species. In addition, the adsorption energy of GLYA on the PtRu (111) surface (-11.2 kcal/mol) was notably lower than that (-26.30 kcal/mol) on Pt (111), which facilitated the desorption of GLYA while inhibiting C−C bond cleavage and deep oxidation. As a result, the Pt0.8Ru0.8/MCM-41 catalyst achieved a GLYA selectivity of 74.1% at a glycerol conversion of 80.1% [Figure 9]. Dou et al. fabricated Sn-doped Pt/C with tailored Pt/Sn ratios for the selective oxidation of glycerol to GLYA[78]. It is found that the TOF of surface Pt atoms in 2.0% Pt9Sn1 reached 938 h-1, which was three times higher than that (281 h-1) of 2.0% Pt. The authors disclosed that the activity enhancement was ascribed to the improved activation of molecular O2 and deprotonation of O−H bonds by surface SnO species. Additionally, the modification of Pt nanoparticles with Sn improved the stability with no significant loss of activity observed after four cycles. Zhang et al. employed an in situ topological transformation strategy to create a PtZn alloy with a low-coordination interfacial structure (PtZn@ZnTiOx/ZnTiO3)[79]. The PtZn alloying effect enhanced the activation of the C−H bonds in glycerol and reduced the activation energy for the reaction (from 40.23 to 25.83 kJ/mol). VO at the low-coordination interface ZnTiOx promoted oxygen activation while optimizing the adsorption mode of glyceraldehyde as intermediate (predominantly bi-dentate coordination) and accelerating its oxidation to GLYA. The catalyst maintained a glycerol conversion of 76.2% and a GLYA selectivity of 61.3% after five cycles. Landge et al. developed a flower-like Janus Pt-Cu/C by nucleating and growing Pt on Cu using a seed-mediated method[80]. By leveraging the anisotropic nature of PtCu bimetallic structure, a synergistic catalysis of Pt and Cu, in which Pt was responsible for the main oxidation reaction and Cu acted as a promoter by facilitating the dehydrogenation reaction, was achieved, resulting in a selectivity of 65% to GLYA at a glycerol conversion of 96%.
Figure 9. Proposed oxidation mechanism over PtRu/MCM-41 in the glycerol oxidation to GLYA[77]. Copyright 2019, Elsevier B.V. GLYA: Glyceric acid.
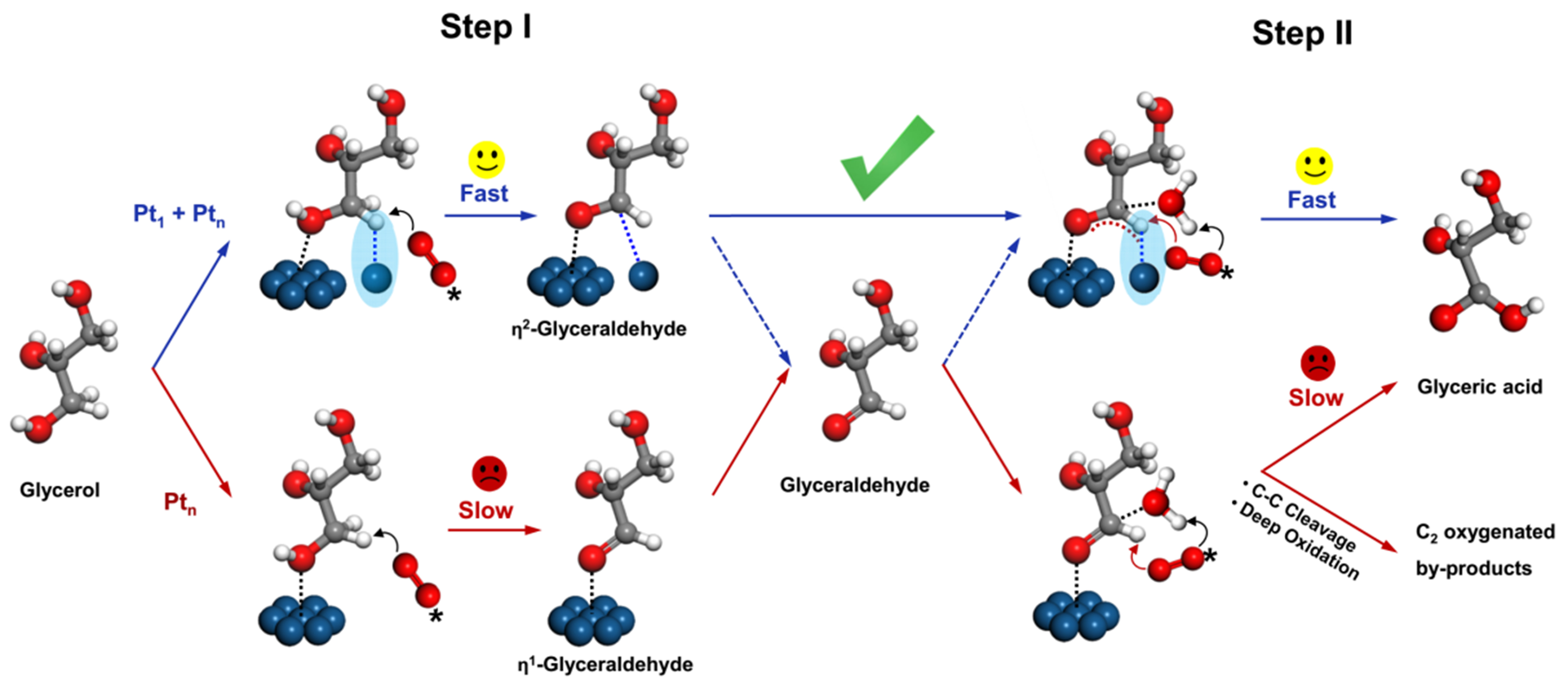
Lately, our research group[81] proposed a cascade synergistic catalysis strategy involving atomic and low-coordinated cluster Pt on well-defined Cu-CuZrOx. Specifically, during the oxidation of glycerol to glyceraldehyde, the C–H activation by atomic Pt1 and O–H activation by cluster Ptn was significantly enhanced, and cluster Ptn for C=O activation followed by O–H insertion and atomic Pt1 for C–H activation in the tandem oxidation of glyceraldehyde to GLYA [Figure 10]. In essence, the enhanced C–H activation in the cascade process by atomic Pt1 was verified to be necessary for the high glycerol activity of 90.0% ± 0.1% and the selectivity of 80.2% ± 0.2% to GLYA.
Figure 10. Proposed catalytic mechanism on the cascade synergistic catalysis over atomic Pt1 and cluster Ptn sites for the selective oxidation of glycerol to GLYA[81]. Copyright 2022, Nature Communications. GLYA: Glyceric acid.
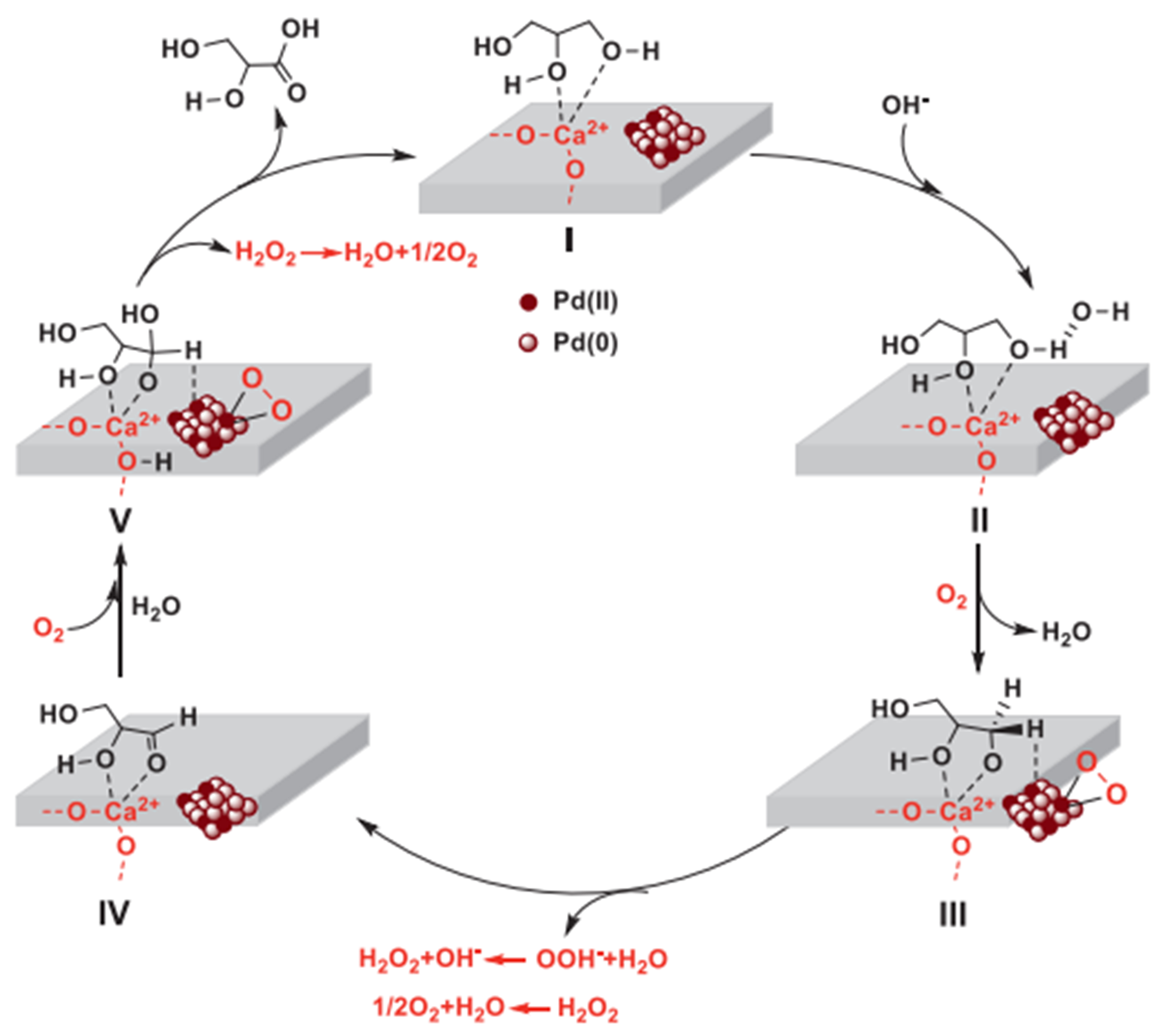
Pd, a member of the Pt group metals, has been considered as a significant research subject due to its more abundant reserves-approximately two to three times that of Pt-similar electronic structure, and comparable catalytic performance to Pt[82]. Li et al. developed supported Pd nanoparticles on hydroxyapatite (HAP), exhibiting a remarkable selectivity of 90% to GLYA at a glycerol conversion of 59%[83]. It revealed that the strong surface basicity of HAP and the high dispersion of Pd(II) and Pd(0) species on the surface effectively inhibited the C–C bond cleavage and/or over-oxidation, thereby improving the selectivity to GLYA. More significantly, the coordination between the Ca2+ sites within the HAP framework and both the primary and secondary C–OH groups of glycerol and played a key role in the direct conversion of the primary C–OH to COOH groups with the secondary C–OH groups remaining intact during the oxidation [Figure 11].
Figure 11. Proposed catalytic mechanism for the selective oxidation of glycerol to GLYA over HAP supporting Pd nanoparticles[83]. Copyright 2022, Institute of Process Engineering. GLYA: Glyceric acid; HAP: hydroxyapatite.
Vajíček et al. fabricated PdBi nanoparticles on gel-based strongly basic anion exchange resins for the selective oxidation of glycerol in the presence of O2 at atmospheric pressure[84]. Supported 3%Pd-1%Bi where the Bi was deposited on the Pd particles (3.4 nm) exhibited a glycerol conversion of 95% and a GLYA selectivity of 68%, which benefited from the geometrical effect involved the partial coverage of the Pd sites by Bi sites, thereby preventing deep oxidation.
Gas-solid phase catalytic oxidation
In addition to the typical liquid-solid phase reaction, the selective oxidation of glycerol in the gas-solid phase reaction at high temperature (300 oC) has been reported. Kumar et al. prepared Au/AC and explored the influence of Au particle size, loading content, and reaction conditions towards the catalytic performance[85]. A 1.0 wt% Au/AC catalyst with a Au particle size of ~6.8 nm and a specific surface area of 1,210 m2/g achieved the best catalytic performance in a selectivity to GLYA of 80% at a glycerol conversion of 82%. However, the gas-solid phase reaction at high temperatures has been reported only in this study. The adsorption and activation of glycerol and the generation of oxygen species might differ from those in the liquid-solid phase reaction. The detailed mechanism still requires further investigation.
To sum up, Table 2 summarized the typical catalysts discussed above with their catalytic performances in the selective oxidation of glycerol to GLYA under specific conditions. Most reports on Pt- and Pd-based monometallic and bimetallic catalysts present a relatively high glycerol conversion of > 80% but a moderate GLYA selectivity, suffering from the activation of the secondary C-OH and the deep oxidation and C-C cleavage. Moreover, the catalyst stability is another problem due to the destruction by the acid products, which has not been referred to in most reports. Poison-resistant systems, such as Pd@Au core–shell structures, could provide the possibility to enhance the stability.
Typical catalysts used for the selective oxidation of glycerol to GLYA
| Catalysts | Reaction conditions | Conv. (%) | GLYA selectivity (%) | Ref. |
| Cubic-Pt/CNT | 0.5 M glycerol, glycerol/Pt (molar) = 212, 60 °C, O2 150 cm3/min, 6 h | 51.5 | 37.9 | [64] |
| Pt/C | 1.0 M glycerol, glycerol/Pt (molar) = 424, 60 °C, O2 150 cm3/min, 6 h | 50.0 | 47.4 | [65] |
| 1% Pt/N-CNTs | 0.1 M glycerol, glycerol/Pt (molar) = 500, 60 °C, O2 150 mL/min, 4 h | 76.1 | 55.6 | [69] |
| Pt/K-AMC-600 | 0.1 M glycerol, glycerol/Pt (molar) = 250, 25 °C, O2 20 mL/min, 8 h | 67.6 | 78.3 | [70] |
| Re-Mg8Al1-LDH-Pt | 0.1 M glycerol, glycerol/Pt (molar) = 1,000, 30 °C, Air 1 atm, 16 h | 87.6 | 66.9 | [71] |
| Pt/ZrO2 | 0.1 M glycerol, glycerol/Pt (molar) = 505, 60 °C, O2 10 bar, 500 rpm, 6 h | 92.9 | 64.9 | [72] |
| Pt/Ca-MCM-41 | 0.2 M glycerol, glycerol/Pt (molar) = 286, 60 °C, O2 10 bar, 1,000 rpm, 8 h | 65.9 | 85.7 | [73] |
| Pt/MnO2/CNT | 0.1 M glycerol, glycerol/Pt (molar) = 1,000, 60 °C, O2 150 mL/min, 2 h | 80.4 | 63.7 | [74] |
| Pt-CeO2/CNT | 0.5 M glycerol, glycerol/Pt (molar) = 200, 60 °C, O2 150 mL/min, 6 h | 88.0 | 69.0 | [75] |
| Au1Pt1/MgO/SBA-15 | 0.2 M glycerol, glycerol/Pt (molar) = 536, 60 °C, O2 10 bar, 8 h | 63.2 | 65.9 | [76] |
| Pt0.8Ru0.8/MCM-41 | 0.2 M glycerol, glycerol/Pt (molar) = 670, 60 °C, O2 10 bar, 1,000 rpm, 12 h | 78.2 | 80.4 | [77] |
| 2.0%Pt9Sn1/C-R | 0.5 M glycerol, glycerol/Pt (molar) = 1,000, 60 °C, O2 15 mL/min, 8 h | 91.1 | 55.4 | [78] |
| PtZn@ZnTiOx/ZnTiO3 | 0.3 M glycerol, glycerol/Pt (molar) = 500, 80 °C, O2 1 bar, 8 h | 80.6 | 65.4 | [79] |
| Pt−Cu/C | 0.2 M glycerol, glycerol/Pt (molar) = 20, 90 °C, O2 1 bar, 700 rpm, 1 h | 96.0 | 65.0 | [80] |
| 0.9%Pt1 + Ptn/Cu-CuZrOx | 0.1 M glycerol, glycerol/Pt (molar) = 300, 60 °C, O2 30 mL/min, 5 h | 90.0 | 80.2 | [81] |
| Pd/HAP-NO | 0.2 M glycerol, glycerol/Pd (molar) = 1,915, 80 °C, O2 10 bar, 5 h | 59.3 | 90.1 | [83] |
| 3%Pd-1%Bi | 0.2 M glycerol, glycerol/Pd (molar) = 591, 50 °C, O2 30 mL/min, 400 rpm, 5 h | 95.0 | 68.0 | [84] |
| 1.0 wt% Au/AC | 1.1 M glycerol, glycerol/Au (molar) = 108, 300 °C, WHSV = 2.52 h-1, 2 h | 82.2 | 80.0 | [85] |
| Pt/Zr@NPCNs | 0.3 M glycerol, glycerol/Pt (molar) = 500, 60 °C, O2 10 bar, 6 h | 63.1 | 81.6 | [86] |
| Pt/CeO2-ZrO2-Fe2O3/SBA-16 | 0.3 M glycerol, glycerol/Pt (molar) = 500, 60 °C, O2 10 bar, 10 h | 99.2 | 68.2 | [87] |
| Pt/Si-C | 0.1 M glycerol, glycerol/Pt (molar) = 143, 60 °C O2 1 atm, 10 h | 74.4 | 86.7 | [88] |
| Pt/biochar | 0.1 M glycerol, glycerol/Pt (molar) = 488, 80 °C, O2 3 bar, 2 h | 82.9 | 86.0 | [89] |
| Pt/ZnO-MCM-41 | 0.1 M glycerol, glycerol/Pt (molar) = 130, 80 °C, O2 10 bar, 1 h | 80.4 | 84.6 | [90] |
CONCLUSION AND OUTLOOK
Glycerol emerges as an exceptionally promising feedstock, offering significant economic benefits and serving as a fundamental subject for catalytic reaction research. Its catalytic oxidation paves the way for the synthesis of a multitude of high-value-added products, which are pivotal for enhancing the sustainability and competitiveness within the biodiesel industry. In this work, we provide a concise review of catalyst design, catalytic mechanisms, and reaction pathways for the production of highly desirable value-added chemicals (DHA/GLYA). The focus is on Au, Pt, and Pd-based catalysts, which have demonstrated remarkable efficacy in these two transformation pathways. The influence of the key structural factors on glycerol oxidation has been meticulously scrutinized, encompassing the size effect of catalysts, the alloy effect, the acidity and basicity of supports, the metal-support interface effect and so on. However, in the aqueous oxidation of glycerol, the relationship between glycerol conversion and DHA/GLYA selectivity operates in a seesaw relationship, achieving simultaneously high conversion and high selectivity for DHA or GLYA still remains a great challenge. For the DHA production, Au-based catalysts offer superior selectivity but require further interfacial engineering to boost overall efficiency. Meanwhile, Pt-based catalysts exhibit high activity yet need modifications to curtail side reactions. For the GLYA production, Pt- or Pd- based catalysts deliver outstanding activity, but endure over oxidation and C-C cleavage while leading to low selectivity. Despite significant progress in the selective oxidation of glycerol to high-value products in recent years, several critical issues and challenges still warrant further investigation. Herein, we propose to explore some research directions in detail, as follows:
(1) Elucidation on the catalytic mechanism of glycerol oxidation by integrating in situ characterization and theoretical calculations. For instance, efforts should focus on probing the activation pathway of glycerol adsorption and intermediate formation using in situ FTIR, investigating the generation of reactive oxygen species using in situ electron paramagnetic resonance (EPR), and simulating key transition state energy barriers using DFT.
(2) Stability enhancement of the heterogeneous catalysts suffering from the nanoparticle agglomeration and metal leaching during the oxidation. For example, emphasis should be placed on the design and precise construction of core-shell encapsulated structures or on enhancing support–metal interactions to stabilize metal particles.
(3) Efficient separation technology to facilitate the large-scale synthesis of the specific value-added chemical from glycerol in industrial production. For instance, attention should be given to ion exchange resin adsorption, distillation–crystallization, and membrane separation techniques.
Moreover, it is worth mentioning that to break the seesaw relationship between the glycerol conversion and the selectivity of specific oxidation product the coupling of the thermal catalysis and the electro-/photo-/photoelectro-catalysis is a promising opportunity. Electrocatalysis enables precise control of the reaction pathway through electrode potential, facilitating atom-economical conversions. Photocatalysis, exemplified by TiO2, utilizes photogenerated supports to drive reactions under mild conditions by reducing the energy consumption. However, they are often limited by the low efficiency compared to the traditional thermal catalysis. Therefore, strategies on the photothermal coupling and electrochemical assistance could achieve the targeted breakthroughs in overcoming thermodynamic barriers at the molecular scale by integrating various energy input modes. Moreover, the coupling of external fields with thermal catalysis, such as CO2 electro-reduction to formate (HCOO-) and glycerol electro-oxidation, is another powerful strategy to enhance material recycling and energy efficiency[91].
DECLARATIONS
Authors’ contributions
Conceptualized and supervised the project: An, Z.; He, J.
Writing - original draft: Chen, T.
Writing - review and editing: An, Z.; He, J.
Supervision and validation: Zhang, J.; Zhu, Y.; Song, H.
Availability of data and materials
Not applicable.
Financial support and sponsorship
Financial support from the National Key R&D Program of China (2022YFB3805602) and the National Natural Science Foundation of China (22138001, 22288102) is acknowledged.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Bagheri, S.; Julkapli, N. M.; Yehye, W. A. Catalytic conversion of biodiesel derived raw glycerol to value added products. Renew. Sustain. Energy. Rev. 2015, 41, 113-27.
2. Wang, H.; Li, H.; Lee, C. K.; Mat Nanyan, N. S.; Tay, G. S. A systematic review on utilization of biodiesel-derived crude glycerol in sustainable polymers preparation. Int. J. Biol. Macromol. 2024, 261, 129536.
3. Chong, C. C.; Aqsha, A.; Ayoub, M.; et al. A review over the role of catalysts for selective short-chain polyglycerol production from biodiesel derived waste glycerol. Environ. Technol. Innov. 2020, 19, 100859.
4. Alazaiza, M. Y. D.; Ahmad, Z.; Albahnasawi, A.; Nassani, D. E.; Alenezi, R. A. Biomass processing technologies for bioenergy production: factors for future global market. Int. J. Environ. Sci. Technol. 2024, 21, 2307-24.
5. Singh, N.; Nyuur, R.; Richmond, B. Renewable energy development as a driver of economic growth: evidence from multivariate panel data analysis. Sustainability 2019, 11, 2418.
6. Husna, M.; Tabak, Y.; Yıldız, M. Glycerol as a feedstock for chemical synthesis. ChemBioEng. Rev. 2024, 11, e202400010.
7. Yang, L.; Li, X.; Chen, P.; Hou, Z. Selective oxidation of glycerol in a base-free aqueous solution: a short review. Chin. J. Catal. 2019, 40, 1020-34.
8. Hu, X.; Lu, J.; Liu, Y.; Chen, L.; Zhang, X.; Wang, H. Sustainable catalytic oxidation of glycerol: a review. Environ. Chem. Lett. 2023, 21, 2825-61.
9. Dodekatos, G.; Schünemann, S.; Tüysüz, H. Recent advances in thermo-, photo-, and electrocatalytic glycerol oxidation. ACS. Catal. 2018, 8, 6301-33.
10. Wang, Y.; Zhou, J.; Guo, X. Catalytic hydrogenolysis of glycerol to propanediols: a review. RSC. Adv. 2015, 5, 74611-28.
11. da Silva Ruy, A. D.; de Brito Alves, R. M.; Reis Hewer, T. L.; de Aguiar Pontes, D.; Gomes Teixeira, L. S.; Magalhães Pontes, L. A. Catalysts for glycerol hydrogenolysis to 1,3-propanediol: a review of chemical routes and market. Catal. Today. 2021, 381, 243-53.
12. Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol hydrogenolysis into useful C3 chemicals. Appl. Catal. B. Environ. 2016, 193, 75-92.
13. Abdullah, A.; Zuhairi Abdullah, A.; Ahmed, M.; et al. A review on recent developments and progress in sustainable acrolein production through catalytic dehydration of bio-renewable glycerol. J. Clean. Prod. 2022, 341, 130876.
14. Basu, S.; Sen, A. K. A review on catalytic dehydration of glycerol to acetol. ChemBioEng. Rev. 2021, 8, 633-53.
15. Xie, Q.; Li, S.; Gong, R.; et al. Microwave-assisted catalytic dehydration of glycerol for sustainable production of acrolein over a microwave absorbing catalyst. Appl. Catal. B. Environ. 2019, 243, 455-62.
16. Keogh, J.; Inrirai, P.; Artioli, N.; Manyar, H. Nanostructured solid/liquid acid catalysts for glycerol esterification: the key to convert liability into assets. Nanomaterials 2024, 14, 615.
17. Perez, F. M.; Gatti, M. N.; Santori, G. F.; Pompeo, F. Transformations of glycerol into high-value-added chemical products: ketalization and esterification reactions. Reactions 2023, 4, 569-634.
18. Zhou, D.; Wang, L.; Chen, X.; et al. Reaction mechanism investigation on the esterification of rosin with glycerol over annealed Fe3O4/MOF-5 via kinetics and TGA-FTIR analysis. Chem. Eng. J. 2020, 401, 126024.
19. Chen, X.; Shu, X.; Zhu, Y.; et al. Highly dispersed MgInCe-mixed metal oxides catalyzed direct carbonylation of glycerol and CO2 into glycerol carbonate. Chin. J. Chem. Eng. 2024, 72, 153-63.
20. Ke, Y.; Xu, H.; Wang, X.; Liu, H.; Yuan, H. Production of glycerol carbonate by coupling glycerol and CO2 over various metal oxide catalyst. J. CO2. Util. 2024, 83, 102813.
21. Wang, Z.; Guo, S.; Wang, Z.; Li, F.; Xue, W.; Wang, Y. A highly efficient rod-like-CeO2-supported palladium catalyst for the oxidative carbonylation of glycerol to glycerol carbonate. RSC. Adv. 2021, 11, 17072-9.
22. Kang, Z.; Shen, Y.; Zheng, Y.; et al. High-active CoPi-Ov nanolayer promotes photoelectrochemical glycerol oxidation for efficient dihydroxyacetone production. Chem. Eng. J. 2025, 515, 163904.
23. Su, K.; Ren, S.; Gao, R. T.; Bai, G. E.; Wu, L.; Wang, L. Bias-free solar-driven ammonia coupled to C3-dihydroxyacetone production through photoelectrochemistry. Angew. Chem. Int. Ed. Engl. 2025, 64, e202422443.
24. Tabassum, N.; Pothu, R.; Pattnaik, A.; et al. Heterogeneous catalysts for conversion of biodiesel-waste glycerol into high-added-value chemicals. Catalysts 2022, 12, 767.
25. Wang, Y.; Xiao, Y.; Xiao, G. Sustainable value-added C3 chemicals from glycerol transformations: a mini review for heterogeneous catalytic processes. Chin. J. Chem. Eng. 2019, 27, 1536-42.
26. Liu, Y.; Zhang, B.; Yan, D.; Xiang, X. Recent advances in the selective oxidation of glycerol to value-added chemicals via photocatalysis/photoelectrocatalysis. Green. Chem. 2024, 26, 2505-24.
27. Liu, B.; Greeley, J. Decomposition pathways of glycerol via C–H, O–H, and C–C bond scission on Pt(111): a density functional theory study. J. Phys. Chem. C. 2011, 115, 19702-9.
28. Meng, F.; Yan, H.; Su, Y.; et al. Unsaturated Fe–Ov–Ti5c structure with enhanced C–H and C–C bond activation ability for selective oxidation of glycerol to glycolic acid. ACS. Sustainable. Chem. Eng. 2024, 12, 10239-51.
29. Koranian, P.; Huang, Q.; Dalai, A. K.; Sammynaiken, R. Chemicals production from glycerol through heterogeneous catalysis: a review. Catalysts 2022, 12, 897.
30. Kong, P. S.; Aroua, M. K.; Daud, W. M. A. W. Conversion of crude and pure glycerol into derivatives: a feasibility evaluation. Renew. Sustain. Energy. Rev. 2016, 63, 533-55.
31. Vitulano, F.; Uggeri, F.; Lattuada, L.; Minguzzi, A.; Vertova, A. Tackling electrocatalytic oxidation of glycerol to dihydroxyacetone: a comprehensive review. Curr. Opin. Electrochem. 2025, 51, 101665.
32. Chen, W.; Zhang, L.; Xu, L.; et al. Pulse potential mediated selectivity for the electrocatalytic oxidation of glycerol to glyceric acid. Nat. Commun. 2024, 15, 2420.
33. Xia, Z.; Ma, C.; Fan, Y.; et al. Vacancy optimized coordination on nickel oxide for selective electrocatalytic oxidation of glycerol. ACS. Catal. 2024, 14, 1930-8.
34. Wen, L.; Zhang, X.; Abdi, F. F. Photoelectrochemical glycerol oxidation as a sustainable and valuable technology. Mater. Today. Energy. 2024, 44, 101648.
35. Yang, Y.; Nalesso, M.; Basagni, A.; et al. Photocatalytic oxidation of glycerol with red light employing quinacridone sensitized TiO2 nanoparticles. J. Mater. Chem. A. Mater. 2025, 13, 18436-44.
36. Liu, X.; Zou, Y.; Jiang, J. Sunlight-driven selective oxidation of glycerol on formate oxidase mimicking AuPt/TiO2. Appl. Catal. B. Environ. Energy. 2024, 350, 123927.
37. Ripoll, M.; Jackson, E.; Trelles, J. A.; Betancor, L. Dihydroxyacetone production via heterogeneous biotransformations of crude glycerol. J. Biotechnol. 2021, 340, 102-9.
38. Meng, Y.; Zou, S.; Zhou, Y.; et al. Activating molecular oxygen by Au/ZnO to selectively oxidize glycerol to dihydroxyacetone. Catal. Sci. Technol. 2018, 8, 2524-8.
39. Liu, S.; Sun, K.; Xu, B. Specific selectivity of Au-catalyzed oxidation of glycerol and other C3-polyols in water without the presence of a base. ACS. Catal. 2014, 4, 2226-30.
40. Yuan, Z.; Gao, Z.; Xu, B. Acid-base property of the supporting material controls the selectivity of Au catalyst for glycerol oxidation in base-free water. Chin. J. Catal. 2015, 36, 1543-51.
41. Yin, Y.; Tang, T.; Xu, C. Au/CuMgAl-hydrotalcite catalysts promoted by Cu+ and basic sites for selective oxidation of glycerol to dihydroxyacetone. Gold. Bull. 2017, 50, 319-26.
42. Wang, Y.; Liu, W.; Zhao, J.; Wang, Z.; Zhao, N. Oxidation of glycerol to dihydroxyacetone over highly stable Au catalysts supported on mineral-derived CuO-ZnO mixed oxide. Appl. Catal. A. Gen. 2024, 671, 119578.
43. Wang, Y.; Pu, Y.; Yuan, D.; et al. Selective oxidation of glycerol to dihydroxyacetone over Au/CuxZr1-xOy catalysts in base-free conditions. ACS. Appl. Mater. Interfaces. 2019, 11, 44058-68.
44. Xiong, Z.; Zhang, X.; Huang, J.; et al. Flower-shaped zinc oxide nanostructures loaded with Au nanoparticles for efficient and highly stable production of dihydroxyacetone from glycerol oxidation. Adv. Sustain. Syst. 2025, 9, 2400947.
45. Pan, Y.; Wu, G.; He, Y.; Feng, J.; Li, D. Identification of the Au/ZnO interface as the specific active site for the selective oxidation of the secondary alcohol group in glycerol. J. Catal. 2019, 369, 222-32.
46. Wu, G.; Zhao, G.; Sun, J.; et al. The effect of oxygen vacancies in ZnO at an Au/ZnO interface on its catalytic selective oxidation of glycerol. J. Catal. 2019, 377, 271-82.
47. Yuan, Z.; Wang, Y.; Xie, W.; et al. Manipulating the interfacial integration mode of a bio-templated porous ZSM-5 platform with an Au/CuZnOx catalyst for enhanced efficiency and recycling stability in glycerol conversion to 1,3-dihydroxyacetone. Nanoscale 2025, 17, 5259-69.
48. An, Z.; Ma, H.; Han, H.; et al. Insights into the multiple synergies of supports in the selective oxidation of glycerol to dihydroxyacetone: layered double hydroxide supported Au. ACS. Catal. 2020, 10, 12437-53.
49. Zhao, G.; Wu, G.; Liu, Y.; He, Y.; Feng, J.; Li, D. Preparation of AuPd/ZnO–CuO for the directional oxidation of glycerol to DHA. Catal. Sci. Technol. 2020, 10, 6223-34.
50. Zhao, M.; Yan, H.; Lu, R.; et al. Insight into the selective oxidation mechanism of glycerol to 1, 3-dihydroxyacetone over AuCu–ZnO interface. AIChE. J. 2022, 68, e17833.
51. Ning, X.; Li, Y.; Yu, H.; Peng, F.; Wang, H.; Yang, Y. Promoting role of bismuth and antimony on Pt catalysts for the selective oxidation of glycerol to dihydroxyacetone. J. Catal. 2016, 335, 95-104.
52. Xiao, Y.; Greeley, J.; Varma, A.; Zhao, Z.; Xiao, G. An experimental and theoretical study of glycerol oxidation to 1,3-dihydroxyacetone over bimetallic Pt-Bi catalysts. AIChE. J. 2017, 63, 705-15.
53. Feng, S.; Yi, J.; Miura, H.; Nakatani, N.; Hada, M.; Shishido, T. Experimental and theoretical investigation of the role of bismuth in promoting the selective oxidation of glycerol over supported Pt–Bi catalyst under mild conditions. ACS. Catal. 2020, 10, 6071-83.
54. Tang, T.; Zhang, H.; Wang, H.; et al. Sub-2 nm PtBi alloy nanoparticles on Bi-N-C single-atom catalyst for selective oxidation of glycerol to 1,3-dihydroxyacetone. Chem. Synth. 2025, 5, 28.
55. Yu, Z.; Fang, J.; Chen, W.; et al. Construction of a uniform Pt-Bi2O3 interface to enhance selective oxidation of glycerol to dihydroxyacetone. Chem. Eng. Sci. 2025, 304, 121113.
56. Duan, X.; Zhang, Y.; Pan, M.; et al. SbOx-promoted pt nanoparticles supported on CNTs as catalysts for base-free oxidation of glycerol to dihydroxyacetone. AIChE. J. 2018, 64, 3979-87.
57. Hirasawa, S.; Watanabe, H.; Kizuka, T.; Nakagawa, Y.; Tomishige, K. Performance, structure and mechanism of Pd–Ag alloy catalyst for selective oxidation of glycerol to dihydroxyacetone. J. Catal. 2013, 300, 205-16.
58. Wang, S.; Li, T.; Li, Y.; Yin, H.; Tian, Z.; Yu, H. Enhanced selective oxidation of glycerol to dihydroxyacetone over Pt@Sn-MFI zeolites. Ind. Eng. Chem. Res. 2024, 63, 16779-88.
59. Huang, N.; Zhang, Z.; Lu, Y.; et al. Assembly of platinum nanoparticles and single-atom bismuth for selective oxidation of glycerol. J. Mater. Chem. A. 2021, 9, 25576-84.
60. Feng, Y.; Bi, Y.; Wang, Y.; et al. Enhanced glycerol oxidation toward dihydroxyacetone over gold/palladium binary nanocatalysts by structure control. Chemistry 2025, 31, e202500601.
61. Cui, L.; Wang, Y.; Yuan, Z.; et al. Benchmarking catalytic activity and cyclic stability of glycerol oxidation to dihydroxyacetone over bio-templated porous ZSM-5 platform composite with Au/CuO. Int. J. Hydrogen. Energy. 2025, 119, 1-12.
62. Yan, H.; Li, S.; Feng, X.; et al. Rational screening of metal catalysts for selective oxidation of glycerol to glyceric acid from microkinetic analysis. AIChE. J. 2023, 69, e17868.
63. Li, Y.; Zaera, F. Factors affecting activity and selectivity in the oxidation of glycerol promoted by platinum catalysts. Catal. Sci. Technol. 2015, 5, 3773-81.
64. Zhou, J.; Hu, J.; Zhang, X.; et al. Facet effect of Pt nanocrystals on catalytical properties toward glycerol oxidation reaction. J. Catal. 2020, 381, 434-42.
65. Liang, D.; Gao, J.; Wang, J.; Chen, P.; Hou, Z.; Zheng, X. Selective oxidation of glycerol in a base-free aqueous solution over different sized Pt catalysts. Catal. Commun. 2009, 10, 1586-90.
66. Li, Y.; Zaera, F. Sensitivity of the glycerol oxidation reaction to the size and shape of the platinum nanoparticles in Pt/SiO2 catalysts. J. Catal. 2015, 326, 116-26.
67. Yan, H.; Yao, S.; Liang, W.; et al. Selective oxidation of glycerol to carboxylic acids on Pt(111) in base-free medium: a periodic density functional theory investigation. Appl. Surf. Sci. 2019, 497, 143661.
68. Meng, Z.; Tran, D.; Hjelm, J.; Kristoffersen, H. H.; Rossmeisl, J. Insight into selectivity differences of glycerol electro-oxidation on Pt(111) and Ag(111). ACS. Catal. 2024, 14, 2455-62.
69. Chen, S.; Qi, P.; Chen, J.; Yuan, Y. Platinum nanoparticles supported on N-doped carbon nanotubes for the selective oxidation of glycerol to glyceric acid in a base-free aqueous solution. RSC. Adv. 2015, 5, 31566-74.
70. Tan, H.; Tall, O. E.; Liu, Z.; et al. Selective oxidation of glycerol to glyceric acid in base-free aqueous solution at room temperature catalyzed by platinum supported on carbon activated with potassium hydroxide. ChemCatChem 2016, 8, 1699-707.
71. Zhang, J.; Li, X.; Xu, M.; et al. Glycerol aerobic oxidation to glyceric acid over Pt/hydrotalcite catalysts at room temperature. Sci. Bull. 2019, 64, 1764-72.
72. Ren, Z.; Li, Y.; Yu, L.; Wang, L.; Yang, Y.; Wei, M. Pt/ZrO2 catalyst with metal-support synergistic effect towards glycerol selective oxidation. Chem. Eng. J. 2023, 468, 143623.
73. Yan, H.; Yao, S.; Zhao, S.; et al. Insight into the basic strength-dependent catalytic performance in aqueous phase oxidation of glycerol to glyceric acid. Chem. Eng. Sci. 2021, 230, 116191.
74. Yang, D.; Liu, X.; Song, F.; et al. Chemoselective oxidation of glycerol over platinum-based catalysts: toward the role of oxide promoter. ChemCatChem 2022, 14, e202200011.
75. Zhang, X.; Zhou, D.; Wang, X.; et al. Overcoming the deactivation of Pt/CNT by introducing CeO2 for selective base-free glycerol-to-glyceric acid oxidation. ACS. Catal. 2020, 10, 3832-7.
76. Liu, Y.; Zha, M.; Qin, H.; et al. Au-promoted Pt nanoparticles supported on MgO/SBA-15 as an efficient catalyst for selective oxidation of glycerol. AIChE. J. 2021, 67, e17196.
77. Yan, H.; Yao, S.; Yin, B.; et al. Synergistic effects of bimetallic PtRu/MCM-41 nanocatalysts for glycerol oxidation in base-free medium: structure and electronic coupling dependent activity. Appl. Catal. B. Environ. 2019, 259, 118070.
78. Dou, J.; Zhang, B.; Liu, H.; et al. Carbon supported Pt9Sn1 nanoparticles as an efficient nanocatalyst for glycerol oxidation. Appl. Catal. B. Environ. 2016, 180, 78-85.
79. Zhang, Y.; Zhang, X.; Yang, P.; Gao, M.; Feng, J.; Li, D. In situ topologically induced PtZn alloy @ ZnTiOx and the synergistic effect on glycerol oxidation. Appl. Catal. B. Environ. 2021, 298, 120634.
80. Landge, V. K.; Sonawane, S. H.; Chaudhari, R. V.; Babu, G. U. B. Selective oxidation of glycerol: a biomass-derived feedstock using the Pt–Cu Janus catalyst for value-added products. Ind. Eng. Chem. Res. 2021, 60, 185-95.
81. An, Z.; Zhang, Z.; Huang, Z.; et al. Pt1 enhanced C-H activation synergistic with Ptn catalysis for glycerol cascade oxidation to glyceric acid. Nat. Commun. 2022, 13, 5467.
82. Wang, C.; Jiang, X.; Wang, Y.; Tang, Y.; Zhou, J.; Fu, G. Recent advances in nonmetallic modulation of palladium-based electrocatalysts. Chem. Synth. 2023, 3, 8.
83. Li, D.; Zhao, X.; Zhou, Q.; et al. Vicinal hydroxyl group-inspired selective oxidation of glycerol to glyceric acid on hydroxyapatite supported Pd catalyst. Green. Energy. Environ. 2022, 7, 691-703.
84. Vajíček, S.; Štolcová, M.; Kaszonyi, A.; et al. Gel-type ion exchange resin stabilized Pd-Bi nanoparticles for the glycerol oxidation in liquid phase. J. Ind. Eng. Chem. 2016, 39, 77-86.
85. Kumar, A.; Belwal, M.; Mohan, V.; Maurya, R. R.; Vishwanathan, V. Catalytic vapor phase oxidation of glycerol to glyceric acid over activated carbon supported gold nanocatalysts. Int. J. Nanosci. 2020, 19, 2050007.
86. Ke, Y.; Zhu, C.; Li, J.; Liu, H.; Yuan, H. Catalytic oxidation of glycerol over Pt supported on MOF-derived carbon nanosheets. ACS. Omega. 2022, 7, 46452-65.
87. Choi, Y. B.; Nunotani, N.; Morita, K.; Imanaka, N. Selective glycerol oxidation to glyceric acid under mild conditions using Pt/CeO2–ZrO2–Fe2O3/SBA-16 catalysts. J. Asian. Ceram. Soc. 2022, 10, 178-87.
88. Huang, X.; Long, Z.; Wang, Z.; Li, S.; Zhang, P.; Leng, Y. Mesoporous silicon-carbon composites: novel supports of platinum nanoparticles for highly efficient selective oxidation of glycerol. Chem. Eng. J. 2023, 470, 144037.
89. Liao, S.; Huang, Q.; Zhou, S.; Gu, Y.; Li, J.; Hu, C. Base-free oxidation of glycerol to glyceric acid over Pt/biochar catalyst. Ind. Crops. Prod. 2025, 229, 120981.
90. Yan, H.; Zhao, M.; Wang, G.; et al. Manipulating Pt-ZnO interface for fast selective oxidation of glycerol to glyceric acid in base-free medium. Chem. Eng. J. 2024, 500, 156842.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].