Type 1 diabetes mellitus: the liver’s role, sex-specific outcomes, and advances in disease-modifying therapies - a literature review
Abstract
Type 1 Diabetes Mellitus (T1DM) is a chronic autoimmune disease characterized by the destruction of pancreatic
Keywords
INTRODUCTION
Type 1 Diabetes Mellitus (T1DM) is a chronic autoimmune disease characterized by the destruction of insulin-producing β-cells in the pancreas, leading to absolute insulin deficiency. Unlike Type 2 Diabetes Mellitus, T1DM primarily manifests during childhood or adolescence, although it can occur at any age. Its etiology involves a complex interplay of genetic predisposition, environmental factors, and immune system dysregulation. Over the past few decades, significant advancements have been made in understanding the pathophysiology, genetic basis, and management of T1DM, yet many challenges remain in achieving optimal disease control and preventing long-term complications.
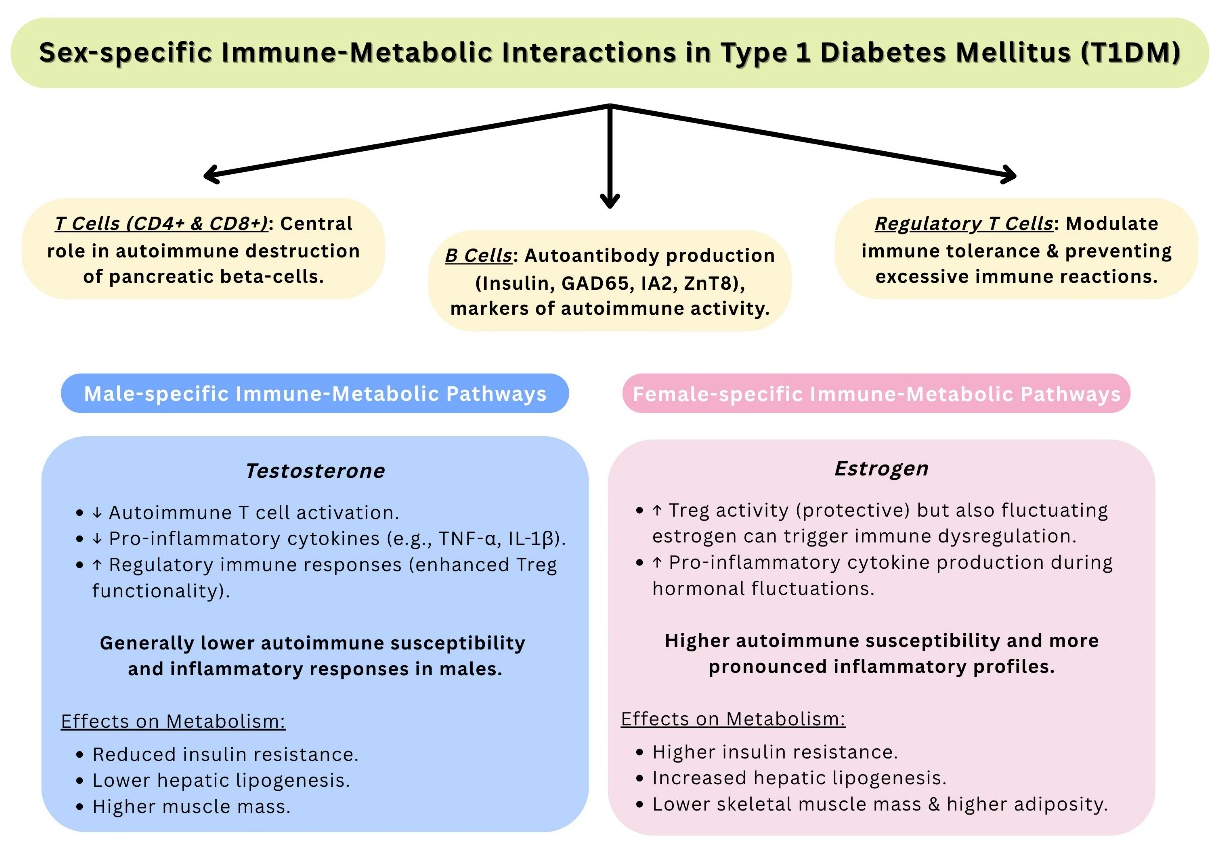
T1DM is distinct among common autoimmune diseases as it does not exhibit a female predominance[1], and studies have highlighted significant sex-based differences in disease presentation, management, and outcomes, underscoring the need for gender-specific research and treatment approaches. Although insulin has remained the primary treatment for T1DM since its discovery, the disease is increasingly recognized as a complex condition with systemic implications, including associations with liver conditions such as metabolic-associated fatty liver disease (MAFLD)[2-4] and metabolic dysfunction-associated steatotic liver disease (MASLD). C-peptide, a byproduct of insulin biosynthesis, is drawing renewed interest for its potential role in understanding and managing T1DM-related complications, suggesting a broader relevance beyond its traditional use as a marker of insulin secretion[5,6]. Emerging disease-modifying therapies, such as tumor necrosis factor-alpha (TNF-α) inhibitors[7-9], anti-CD3 antibodies[9], abatacept, cell-based therapies, and vitamin D supplementation, show promise in slowing disease progression, preserving β-cell function, and improving glycemic control[10].
This literature review aims to synthesize recent research on the epidemiology, sex differences, and risk factors of T1DM, the liver’s role, the involvement of C-peptide, the contribution of T and B cells, and evolving therapeutic strategies, highlighting areas for future investigation and potential clinical applications.
DISCUSSION
Epidemiology and sex differences
T1DM is the only common autoimmune disease that does not exhibit a female predominance[1]. In fact, in Caucasian communities, the prevalence of T1DM is higher among men compared to women[1]. T1DM typically presents in young individuals, with most cases diagnosed before the age of 20[1]. Globally, more than one million children and adolescents are estimated to be affected by T1DM [Table 1][11]. In recent years, the incidence of T1DM has been rising in the United States, particularly among Hispanic and non-Hispanic Black youth [Table 1][12,13].
Summary of epidemiological trends and sex-specific clinical outcomes in Type 1 diabetes mellitus
| Aspect | Key findings | Sex-specific differences |
| Global prevalence[11] | > 1 million children and adolescents affected globally | Some studies report no sex-based difference; others indicate higher prevalence in males |
| Incidence trends[13] | Rising globally, particularly among Hispanic non-Hispanic Black youth in the U.S. | Postprandial C-peptide and insulin levels are notably higher in females |
| Glycemic control[30] | HbA1c targets (< 7%) often unmet | Females tend to have poorer glycemic control and are less likely to meet BP/lipid targets |
| Diabetic complications[25] | Includes CVD, DKD, MAFLD, and MASLD | Females are at higher risk of CVD and renal disease progression, with a delayed onset of DKD |
| Renal disease[15] | DKD affects 30% of patients with T1DM | CKD is more common in females, while ESRD rates are higher in males |
| Cardiovascular risk[24] | T1DM increases CVD risk by up to 30-fold and shortens life expectancy | Women experience greater excess mortality and worse treatment outcomes compared to men |
| Liver involvement (ALT levels)[1] | Elevated ALT observed in ~9.5% of T1DM patients; associated with MAFLD/MASLD | Poorer glycemic control in women may exacerbate liver-related complications |
| Sex hormones & insulin sensitivity[1] | Estrogens/androgens influence β-cell function and immune modulation | Females show higher insulin resistance, likely due to lower muscle mass and increased adiposity |
Despite these trends, studies have reported inconsistent findings regarding sex-based differences in T1DM prevalence. For instance, the SEARCH for Diabetes in Youth study, initiated in 2000 by the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases, found no clear sex differences in T1DM prevalence between 2001 and 2009[14]. However, multiple outcomes related to T1DM appear to be worse in females than in males, especially regarding body mass index (BMI), glycemic control, diabetic ketoacidosis, and quality of life[13]. Additionally, women have been reported to show higher postprandial C-peptide and insulin levels following a meal test[1]. It is also noteworthy that diabetic renal complications tend to manifest ten years later in women compared to men of the same age[15].
Gender medicine emphasizes analyzing biological sex differences and their impact on disease pathophysiology, presentation, and outcomes[16-19]. Many studies on T1DM complications have focused exclusively on one sex, often males, limiting the reliability of findings and complicating cross-sex comparisons[20]. This historical bias has overlooked important sex-specific factors, including differences in renal glucose handling and disease progression[15].
Women generally exhibit greater insulin resistance than age-matched men, which may be explained by lower skeletal muscle mass, higher adipose tissue mass, and increased levels of circulating free fatty acids[1,21,22]. One study examining pregnancy-induced metabolic changes in mice highlighted dynamic alterations in body weight and fat deposition, as well as impaired glucose tolerance and insulin sensitivity during late pregnancy. Thermogenic activity in brown and inguinal white fat was reduced, while gonadal fat showed increased lipid mobilization. Mammary gland differentiation was observed in certain fat depots, and metabolic adaptations were also seen in the liver and pancreas. These findings reflect similar metabolic changes during human pregnancy and may help inform the prevention and treatment of maternal metabolic diseases[20]. Moreover, young females with T1DM may be subject to treatment bias, which could affect the quality of their daily care and the management of risk factors[11]. Thus, thorough analysis and comparison of sex differences are essential for a deeper understanding of T1DM and the optimization of its clinical management and care [Table 1].
Glycemic and blood pressure control, dyslipidemia, sex hormones
Glycemic and blood pressure control
Sex differences play a critical role in glucose homeostasis, particularly in individuals with T1DM and prediabetic syndromes. Women with T1DM are less likely to achieve an HbA1c level < 7%, to be prescribed lipid-lowering medications [Table 1], and, when treated, to reach recommended blood pressure and lipid targets[23]. A recent study demonstrated the impact of sexual dimorphism on mitochondrial bioenergetic adaptations in T1DM[24]. These findings may help explain the sex-specific differences in glycemic control observed in boys but not girls, underscoring the need to develop sex-based therapies for diabetes[1].
Dyslipidemia
Current literature emphasizes that the atherosclerosis process in individuals with T1DM often begins in early childhood at the endothelial level and progresses in an accelerated manner[24]. A 2011 study comparing sex differences in T1DM revealed that the protective effect of high-density lipoprotein (HDL) may be markedly decreased in women with T1DM compared to men, which may help explain the increased risk of complications in female patients[25].
Sex hormones
Sex is increasingly recognized as a significant factor influencing T1DM and warrants greater clinical attention[11]. Several hypotheses attempt to explain the biological and gender-related bases for these variations, although the mechanisms remain incompletely understood. Sexual dimorphism is thought to stem, at least in part, from the influence of sex hormones such as estrogens and androgens. During puberty, hormonal changes in women are associated with better-preserved residual β-cell function compared to men, suggesting a transient protective effect against T1DM[11,26,27]. Furthermore, recent studies have identified an association between sex hormone-binding globulin (SHBG) and T1DM risk. Elevated levels of SHBG in women have been linked to a lower risk of developing diabetes[14]. Therefore, imbalances in sex hormones may represent an important risk factor that merits further consideration.
Cardiovascular system
Cardiovascular complications are also a major determinant of prognosis and quality of life in patients with T1DM [Table 1]. Studies have shown that individuals with T1DM have a 30-fold increased risk of severe cardiovascular disease and a life expectancy that is 16 years shorter than those without T1DM[24]. For instance, data from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort revealed that for every 1% increase in mean HbA1c, the risk of developing any form of cardiovascular disease increased by 31%[24].
Sex-specific analyses indicate a markedly higher risk of cardiovascular disease (CVD) in women with T1DM compared to men[16]. Notably, in individuals with T1DM, the mortality rate from CVD before age 40 is 10 times higher in men, but strikingly 40 times higher in women[24,28,29].
Although studies on T1DM and atrial fibrillation are limited, recent analyses have confirmed a higher incidence of atrial fibrillation in individuals with T1DM[30]. The risk is only slightly elevated in men, but is significantly higher (~50%) in females of the same age group[30].
A longitudinal study spanning 11 years and involving 774 individuals with T1DM found that the Fatty Liver Index (FLI), a non-invasive marker of liver steatosis, independently predicted all-cause mortality and major cardiovascular events. Patients with an FLI ≥ 60 had significantly higher risks of death and cardiovascular complications, highlighting the importance of liver health assessment in T1DM management to reduce adverse outcomes[31].
Regarding heart failure, a meta-analysis of 47 cohort studies including over 12 million individuals found that T1DM and T2DM confer a higher risk of heart failure in women than in men, with women showing a 47% greater excess risk[16]. Several factors may contribute to this disparity, including: (1) poorer glycemic control in women with T1DM; (2) undertreatment of women with diabetes, which may lead to diabetic cardiomyopathy; and (3) a longer duration of prediabetes in women, as they were found to have two additional years of hyperglycemia compared to men[16].
Several narrative reviews have highlighted the critical link between liver dysfunction, particularly MASLD, and increased cardiovascular risk in patients with T1DM. These reviews emphasize the importance of early detection and targeted management of liver abnormalities as essential strategies to improve cardiometabolic outcomes in this population[32-34].
Renal failure and diabetic kidney disease
Diabetic Kidney Disease (DKD) is one of the most common complications of diabetes mellitus [Table 1], affecting approximately 30% of individuals with T1DM[14]. An epidemiological study highlights the importance of achieving better glycemic control to reduce the incidence of DKD in patients with T1DM or T2DM, regardless of sex[35]. While End-Stage Renal Disease (ESRD) is more prevalent in men, CKD appears to be more common in women[15]. Research suggests that women with T1DM may be at increased risk of developing CKD, possibly due to greater susceptibility to changes in glomerular filtration rate (GFR)[14].
Recent investigations into sex differences across renal transporters throughout the nephron have shown that sodium-glucose co-transporters 1 and 2 (SGLT1 and SGLT2) function in a sex-independent manner. This may explain why sex differences are not consistently reported in studies evaluating the efficacy of currently available SGLT2 inhibitors[14]. However, several studies have reported a higher incidence of adverse effects in women with T1DM undergoing SGLT2 inhibitor therapy - such as increased rates of urinary tract infections (UTIs), genital infections, and diabetic ketoacidosis - compared to men[14].
Sex hormones and immune regulation in T1DM
Sex differences play a significant role in the pathogenesis and progression of T1DM [Table 1]. Estrogens promote β-cell survival and modulate immune responses by influencing regulatory T cells and cytokine expression, while androgens have complex, dose-dependent effects on insulin sensitivity and inflammation[1]. Estrogen surges during puberty are associated with better residual β-cell function in females than in males[11]. Additionally, elevated SHBG levels in women have been inversely associated with T1DM risk, suggesting a protective endocrine profile[14]. These hormonal factors may partially explain observed sex differences in insulin sensitivity, as females with T1DM often exhibit greater insulin resistance than
Type 1 diabetes mellitus and the role of the liver
Liver Involvement and MAFLD in T1DM
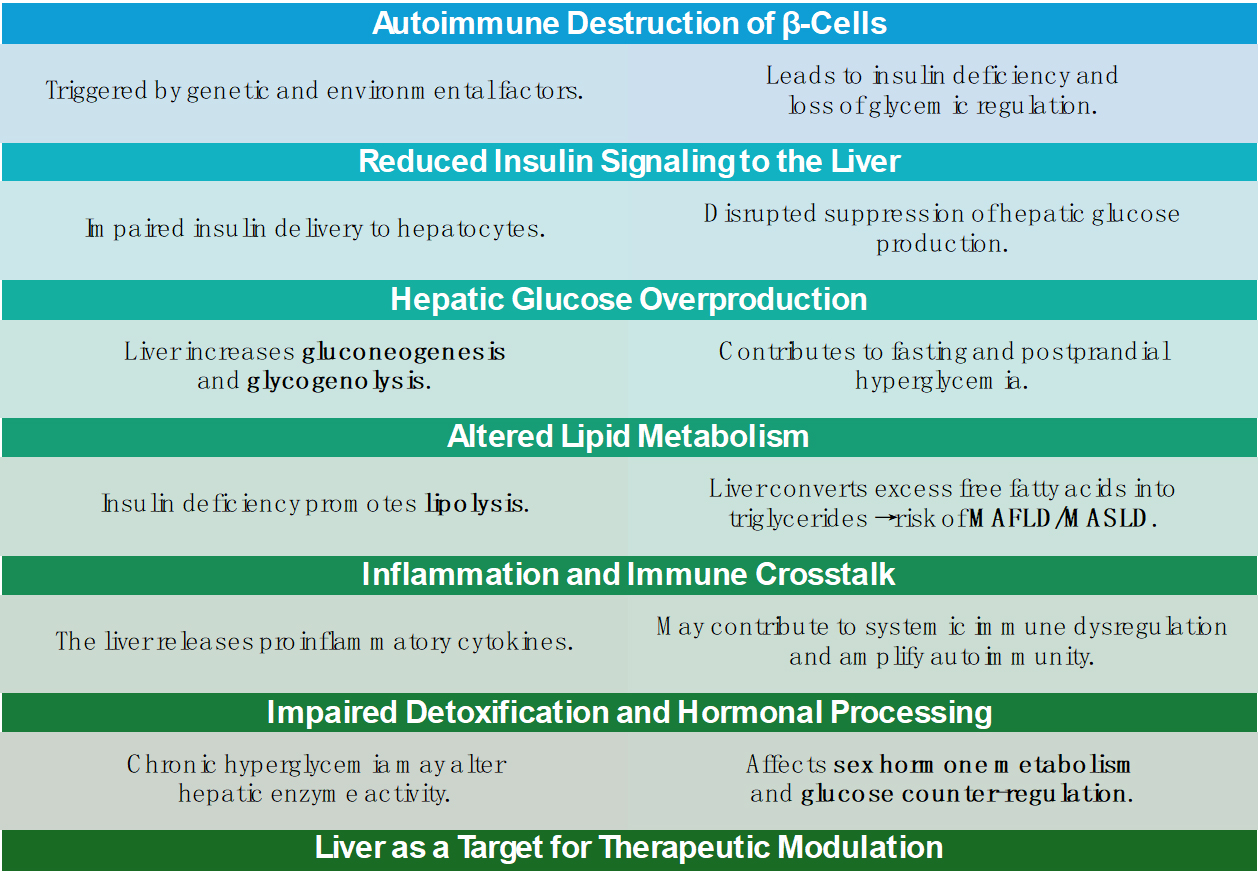
T1DM is characterized by autoimmune destruction of pancreatic β-cells [Figure 2], resulting in absolute insulin deficiency and lifelong dependency on exogenous insulin therapy. Insulin has remained the cornerstone of treatment for over a century since its discovery[36]. Innovations such as hybrid closed-loop systems have significantly improved the quality of life for individuals with T1DM[36]. However, despite these advancements, morbidity and mortality associated with the disease continue to rise globally[31].
Figure 2. Overview of the Liver’s Role in the Pathophysiology and Progression of Type 1 Diabetes Mellitus.
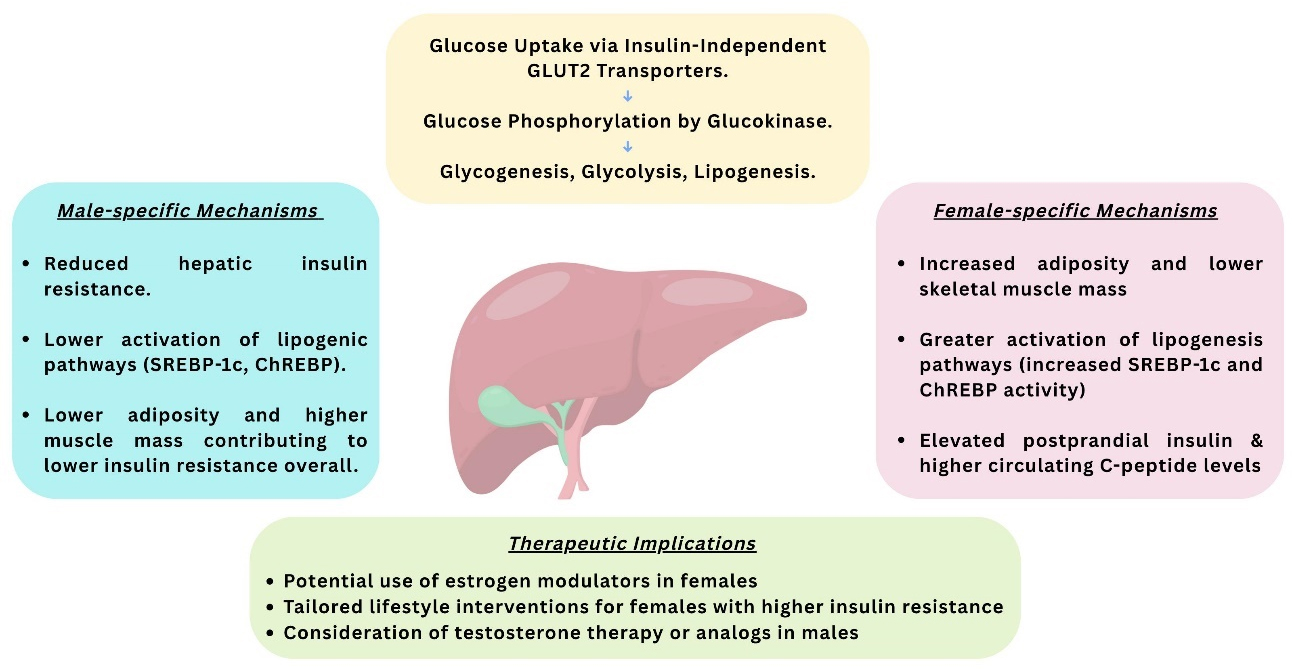
The liver plays a central role in glucose and lipid metabolism and is significantly affected in T1DM due to insulin deficiency and chronic hyperglycemia [Figure 2]. Hepatocytes take up glucose via
Figure 3. Schematic Illustration of the Metabolic Role of the Liver in Hepatic Insulin Resistance and Implications for Therapy.
Emerging evidence indicates early hepatic involvement in the pathogenesis of T1DM. A study analyzing cord serum samples found that reduced levels of major choline-containing phospholipids were associated with an increased risk of developing T1DM, suggesting early liver-based metabolic disruptions[32].
In patients with poorly controlled T1DM, hepatic glycogen metabolism is markedly impaired [Figure 2]. Research has shown significant reductions in both hepatic glycogen synthesis and breakdown in these individuals. Although short-term insulin therapy improved these abnormalities, levels did not return to normal. Furthermore, glycogenic hepatopathy-characterized by excessive hepatic glycogen storage-has been observed in patients with T1DM, particularly in those with erratic glycemic control and frequent insulin fluctuations[40-43]. Beyond glycogen storage, chronic hyperglycemia and exogenous insulin therapy contribute to hepatic steatosis. Mechanistically, this is linked to upregulated GLUT2 expression [Figure 3] and the activation of lipogenic transcription factors such as carbohydrate-responsive element-binding protein (ChREBP) and sterol regulatory element-binding protein 1c (SREBP-1c), which promote hepatic de novo lipogenesis and fat accumulation[44].
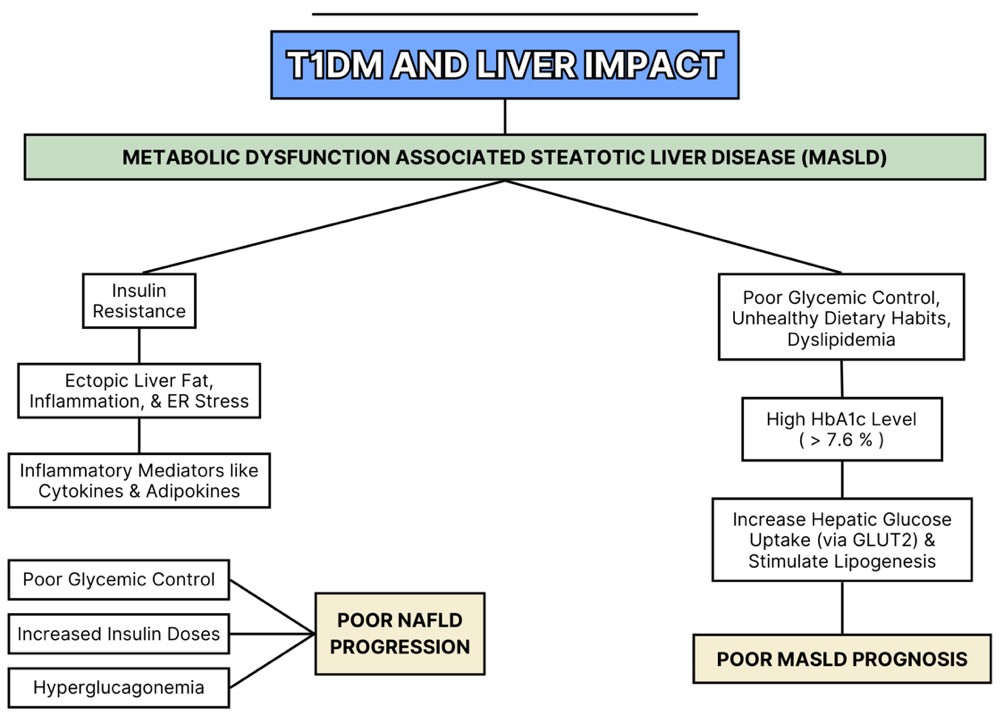
MAFLD, more recently referred to as MASLD, is increasingly reported in T1DM. A systematic review and meta-analysis of 3,901 adults with T1DM reported a MAFLD prevalence of 22%[2]. This high prevalence is attributed to factors such as elevated circulating free fatty acids, systemic inflammation, oxidative stress, and suboptimal glycemic control. Additionally, the total daily insulin dose has been positively correlated with MAFLD progression[45].
T1DM-associated hyperglucagonemia, caused by the absence of intra-islet amylin secretion, further contributes to hepatic lipid accumulation and exacerbates metabolic dysregulation[2]. Elevated levels of alanine aminotransferase (ALT) are observed in approximately 9.5% of individuals with T1DM. In pediatric patients with poor glycemic control, the odds of elevated liver enzymes are more than twice as high compared to well-controlled peers, even after adjusting for confounding factors[46]. These markers serve as clinically relevant, non-invasive indicators of hepatic involvement in T1DM and may help identify high-risk patients[45].
Finally, a Mendelian randomization study has established a causal relationship between T1DM and the progression of liver fibrosis and cirrhosis. It further showed that both acute and chronic complications, especially neurological and ocular, are independently associated with an increased risk of liver fibrosis. These findings underscore the importance of comprehensive T1DM management strategies that include routine monitoring of liver health[47].
Metabolic dysfunction-associated steatotic liver disease and T1DM
A link between MASLD and T1DM has been recently established in novel published studies[31]. These two conditions share common predisposing factors, including obesity and insulin resistance. Consequently, the rising prevalence of obesity among people with T1DM has made the need for intensive insulin therapy increasingly inevitable[31]. Studies investigating the prevalence of MASLD in individuals with T1DM are limited, and existing findings are difficult to compare due to variations in study populations[31].
Several factors in T1DM contribute to the consequent development of MASLD, including poor glycemic control, obesity, dyslipidemia, unhealthy dietary habits, and lipoprotein abnormalities[31,48,49]. Notably, a cross-sectional study of 659 adults with T1DM reported an association between elevated HbA1c levels and MASLD, independent of obesity status[50]. In this study, patients with HbA1c levels above 7.6% showed significantly higher liver enzyme levels. Chronic hyperglycemia in T1DM promotes hepatic glucose uptake via glucose transporter 2 (GLUT2), leading to activation of sterol regulatory element-binding proteins (SREBPs) and carbohydrate-responsive element-binding proteins (ChREBPs)[36]. These pathways stimulate hepatic de novo lipogenesis[31], thereby contributing to the development of MASLD in T1DM patients. Mechanistically, the literature highlights the role of advanced glycation end products (AGEs) in vascular complications. However, it often overlooks the significance of early glycation of Amadori-modified proteins, which have been found to be strongly associated with an increased risk of nephropathy in T1DM patients[45]. Current evidence remains limited and inconclusive, highlighting the need for further research to clarify the liver’s role in T1DM-related complications and its implications for prognosis and clinical management. Liver complications commonly observed in T1DM are illustrated in Figure 4.
Involvement of c-peptide in type 1 diabetes mellitus
Mechanism of action of C-peptide in T1DM
C-peptide is a 31-amino-acid peptide that connects the A and B chains of proinsulin and has emerged as a potentially valuable factor in understanding the pathogenesis of T1DM-related complications. Following cleavage by endoproteases, C-peptide is secreted into the bloodstream in equimolar amounts with endogenous insulin, making it a reliable marker of insulin production[51,52]. C-peptide binds to specific cellular receptors, such as G-protein coupled receptor 146 (GPR146), initiating intracellular signaling cascades that regulate essential endothelial functions, including nitric oxide production, thereby supporting vascular health. In T1DM, vascular damage is partly attributable to endothelial dysfunction; C-peptide’s modulation of these signaling pathways helps mitigate such damage[5,53-55]. Additionally, studies have shown that C-peptide restores sodium-potassium (Na+/K+)-ATPase activity in neurons and glomeruli, contributing to reduced nerve injury and decreased hyperfiltration-induced stress on the kidneys[5,56,57]. In certain studies, an early C-peptide response to glucose challenges suggests better-preserved β-cell function in individuals with single autoantibody positivity. This highlights C-peptide’s role in glucose-stimulated insulin secretion and supports its potential as a biomarker for residual β-cell activity in cases of less severe autoimmunity[58,59].
Evidence linking C-peptide levels to T1DM complications
Diabetic nephropathy, also referred to as DKD, is a chronic microvascular complication of T1DM resulting from hyperglycemia-induced damage to the glomeruli. In the early stages, elevated blood glucose levels lead to an increase in GFR. Over time, heightened intraglomerular and transcapillary pressures cause glomerulosclerosis. In addition, disruptions in renal hemodynamics promote interstitial fibrosis through the release of cytokines and growth factors. DKD is classified into five stages based on the patient’s GFR, ranging from stage 1 (pre-nephropathy) to stage 5 (end-stage renal disease). Maintaining strict glycemic control remains essential in preventing the onset and progression of DKD, underscoring the need to explore therapeutic strategies such as C-peptide administration[5].
In individuals with adult-onset T1DM, measurable levels of C-peptide may persist even after long disease duration, and are associated with improved glycemic control[60]. Furthermore, adults with T1DM typically present with higher C-peptide levels at diagnosis, which may contribute to a reduced incidence of diabetic ketoacidosis and a lower risk of hypoglycemia[60]. C-peptide also exerts anti-inflammatory effects by inhibiting nuclear factor kappa B (NF-κB) - a key pro-inflammatory pathway - and promoting the production of anti-inflammatory cytokines (e.g., IL-10). This anti-inflammatory environment reduces vascular inflammation and oxidative stress, helping to protect against complications like diabetic nephropathy[5]. Beyond renal protection, C-peptide influences critical physiological processes including vascular function, neural integrity, and systemic inflammation in T1DM. These actions have significant implications for understanding disease progression and the development of complications[61-65]. Higher circulating levels of C-peptide may protect against microvascular damage and inflammatory responses, offering a potential therapeutic target in T1DM management[61].
Therapeutic potential of C-peptide in T1DM
Research indicates that C-peptide administration has shown beneficial, albeit clinically limited, effects on kidney-associated dysfunctions. However, the DCCT study provides strong evidence that even residual levels of C-peptide are associated with improved clinical outcomes in patients. Specifically, C-peptide has been shown to alleviate diabetic nephropathy by reducing glomerular hyperfiltration and albuminuria. It modulates endothelial function, enhances nitric oxide production, and inhibits pro-inflammatory pathways, thereby offering renal protection[66-69]. In T1DM models, C-peptide therapy has also improved nerve conduction velocity and reduced neuropathic symptoms. These effects are attributed to its ability to restore Na+/K+-ATPase activity and improve endoneural blood flow[70,71]. Regarding its vascular effects, C-peptide exerts anti-inflammatory effects by downregulating adhesion molecules such as P-selectin and intracellular adhesion molecule-1 (ICAM-1) on endothelial cells, thereby decreasing leukocyte adhesion and vascular inflammation. However, some studies suggest that C-peptide may also have pro-atherogenic effects by stimulating vascular smooth muscle cell proliferation through Src kinase and MAPK pathways[70]. These findings reinforce the notion that C-peptide is not just a biomarker but may also play an active role in mitigating diabetic complications[72,73]. Importantly, these therapeutic utilities must be supported by investigations into C-peptide’s biological activity in both in vitro and in vivo models[5]. In studies on combination therapies, fluctuations in C-peptide levels observed in placebo groups - compared with those receiving combination therapy - suggest that these changes are not solely due to the “honeymoon phase” (a period following T1DM diagnosis during which the pancreas retains some insulin-producing capability) but reflect a real therapeutic effect[74-76]. C-peptide also has potential utility in guiding therapeutic strategies. Insulin monotherapy is generally recommended for individuals with random C-peptide levels below
Despite these promising findings, the utility of C-peptide in predicting therapeutic responses remains limited. One key challenge is its temporal variability-C-peptide levels fluctuate based on disease duration, age, and metabolic state, making cross-patient comparisons difficult. Additionally, C-peptide exhibits delayed responsiveness; its levels may not immediately reflect ongoing β-cell destruction or the effects of immunomodulatory therapy. In early-stage disease or in individuals with a single autoantibody, C-peptide concentrations may remain within normal range despite active immune-mediated β-cell injury, limiting its usefulness in identifying therapeutic targets or predicting disease progression[59].
Finally, researchers are exploring the use of C-peptide in combination with other therapies, which may enhance treatment efficacy and improve the management of T1DM and its complications[61].
Role of T cells and B cells in T1DM
Overview of immune-mediated mechanisms in T1DM
T1DM is a T cell-mediated autoimmune disease characterized by the selective destruction of pancreatic insulin-secreting β-cells, principally by islet-reactive T cells[78-80]. This autoimmune response arises from a complex interplay among genetic susceptibility, environmental factors, and the gastrointestinal (GI) microbiome[74]. For example, viral infections are thought to contribute to β-cell damage, while molecular mimicry - a mechanism associated with the GI microbiome - can trigger T cell responses that lead to T1DM[78]. In addition to T cells, B cells have been detected infiltrating pancreatic islets in humans, where they contribute to disease progression[78]. Studies have shown that B cell-deficient mice do not develop spontaneous T1DM, suggesting a critical role for B cells in disease onset. Similar to T cells, some B cells that develop in the bone marrow can randomly acquire autoreactive breakpoint cluster region (BCR) proteins[81]. Moreover, the activation of self-reactive T cells in T1DM is probably driven by antigen-presenting cells (APCs) - such as dendritic cells (DCs), macrophages, B cells, and islet β-cells - through the presentation of self-antigens via MHC class I or II molecules[78]. Notably, exposure to insulin in the diet during infancy has been shown to trigger both B and T cell responses[82].
Contribution of T cells to T1DM progression
In T1DM, the immune system erroneously identifies pancreatic β-cells as foreign and initiates an autoimmune attack, leading to their destruction. This impairs insulin secretion and disrupts blood glucose regulation, driving disease onset and progression. Antigen-specific immune cells, particularly CD4+ and CD8+ T cells, along with other immune cells such as natural killer cells and macrophages, are activated and recruited to target β-cells[82]. Various types of islet autoantigen-specific T cells have been identified. These include: preproinsulin-specific T cells, which initiate islet destruction; glutamic acid decarboxylase 65 (GAD65)-specific T cells, which trigger inflammation and amplify the autoimmune response; zinc transporter 8 (ZnT8)-specific T cells, which play a critical role in diabetes onset under immune-compromised pancreatic conditions; insulin-specific T cells, involved directly in β-cell destruction; and islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP)-specific T cells, which also contribute to insulitis and β-cell damage[82]. Cytokines play a pivotal role in these mechanisms. For example, interferon gamma (IFN-γ) may modulate the autoimmune response by sustaining T cell activation[82].
Role of B cells in T1DM pathophysiology
During development, B cells expressing autoreactive BCRs may undergo deletion or BCR editing to avoid autoimmunity. In T1DM, however, autoreactive B cells are thought to receive help from activated CD4+ T cells, leading to the production of autoantibodies - primarily targeting insulin, GAD65, IA2, and ZnT8. Although these autoantibodies are not themselves pathogenic, they serve as critical biomarkers for disease prediction and assessment of immune profiles based on the specificity of the targeted antigens. The functional role of autoreactive B cells in human T1DM remains poorly understood, although
Despite their diagnostic utility, autoantibody biomarkers have several limitations. First, they lack pathogenicity - autoantibodies are not directly cytotoxic and do not reliably reflect active β-cell destruction. Second, they offer limited predictive value regarding therapeutic response. For instance, in the Abatacept prevention trial, the drug demonstrated immunomodulatory effects, but did not significantly delay disease progression in antibody-positive individuals[83]. Third, antibody status does not always correlate with disease trajectory; some individuals progress to clinical T1DM even after seroreversion, emphasizing the role of non-humoral mechanisms in pathogenesis.
Type 1 diabetes and the role of inflammation
T1DM is fundamentally an autoimmune disorder characterized by the selective destruction of pancreatic
The resulting inflammatory environment promotes polarization of macrophages to the pro-inflammatory M1 phenotype and facilitates the recruitment and activation of autoreactive CD4+ and CD8+ T cells, which mediate β-cell destruction through both cytokine release and direct cytotoxicity[84]. Chronic inflammation perpetuates β-cell apoptosis and loss of insulin production - hallmark features of T1DM[84]. Genetic factors further modulate this process; for example, polymorphisms in genes like C-type lectin domain family 16 member A (Clec16a), which regulate mitophagy, have been shown to impair mitochondrial quality control and amplify inflammation[84,85]. These insights have spurred the development of novel therapeutic strategies aimed at controlling inflammation and restoring mitophagy. Several pharmacological agents targeting the NLRP3 inflammasome and other inflammatory pathways are currently under investigation[86].
Disease modifying therapies
Approved and near-approval therapies
TNF-α inhibitors
TNF-α has been implicated in the progression of T1DM [Table 2], both by enhancing antigen presentation and through its direct cytotoxic effects[10]. As such, TNF-α is represents a potential therapeutic target for T1D. TNF-α inhibitors function by binding to TNF-α and its receptors, thereby interfering with its pathological activity. Common TNF-α inhibitors include adalimumab, certolizumab, etanercept, and infliximab. Clinical trials investigating TNF-α inhibitors such as etanercept and golimumab in T1DM have shown reductions in HbA1c and increases in C-peptide levels, indicating their therapeutic efficacy[10]. Adalimumab has also been associated with improved glycemic control and reduced hypoglycemia; however, case reports have documented hypoglycemic episodes in some patients[10,87]. These adverse effects underscore the need for close monitoring and further investigation to determine which TNF-α inhibitors provide optimal glycemic control without inducing hypoglycemia [Table 3].
Summary of C-peptide’s role in type 1 diabetes mellitus
| Focus area | Scientific insights | Implications for T1DM |
| Cellular mechanisms | - Activates GPR146 receptors - Stimulates nitric oxide production - Restores Na⁺/K⁺-ATPase in neurons | - Enhances vascular function - Provides neuro- and reno-protection - Counters endothelial dysfunction |
| Diagnostic utility | - Secreted in a 1:1 ratio with insulin - Reflects endogenous insulin secretion - Sensitive in mild autoimmunity | - Assesses β-cell reserve - Helps stratify disease severity - Guides early intervention |
| Microvascular complications | - Reduces inflammation & oxidative stress - Improves glomerular dynamics - Suppresses NF-κB, promotes IL-10 | - May slow progression of nephropathy & neuropathy - Fewer DKA & hypoglycemia episodes - Correlates with better glycemic control |
| Therapeutic modulation | - Supports endothelial/immune function - Lowers ICAM-1 and P-selectin - Promotes smooth muscle proliferation | - Potential as adjunctive therapy - Improves renal & neurological outcomes - Requires monitoring for pro-atherogenic risk |
| Limitations in clinical Application | - Varies with age, disease stage, and metabolic state - Lags behind β-cell destruction - Normal levels may mask early damage | - Not ideal as a standalone biomarker - Best interpreted in clinical context alongside other markers |
| Research & therapeutic outlook | - Preservation of C-peptide (e.g., 20%) lowers HbA1c by 0.5% - Demonstrates utility beyond the honeymoon phase - Supported by placebo-controlled studies | - Encourages personalized therapy thresholds - Supports long-term trials for evaluating sustained benefits |
Summary of emerging disease-modifying therapies in type 1 diabetes mellitus: mechanisms, effects, and sex-based considerations
| Therapy | Mechanism | Effects | Considerations |
| TNF-α inhibitors[89] | Suppress inflammatory cytokines | Lower HbA1c, increase C-peptide | Risk of hypoglycemia; sex-stratified trials needed |
| Anti-CD3 (Teplizumab) | Blocks T cell activation, preserves β-cells | Delays T1DM onset, maintains C-peptide for over 2 years | Females may exhibit differential immune responses |
| Abatacept (CTLA-4 fusion)[90] | Inhibits T cell co-stimulation (CD80/CD86) | Slows β-cell decline in early disease | More effective if initiated early; sex-specific response unclear |
| Treg cell therapy[92] | Restores immune tolerance | Temporarily delays T1DM progression | Irisin may enhance efficacy; lack of sex-specific data |
| MSC therapy[91] | Immunomodulatory; reduces β-cell apoptosis | Reduces HbA1c, increases C-peptide, lowers insulin needs | Some sex-specific adverse events reported (e.g., UTIs in women) |
| Vitamin D supplementation[95] | Enhances immune regulation and β-cell preservation | Mixed results; may reduce insulin autoantibodies | Deficiency more common in females with T1DM |
| C-Peptide therapies[70] | Improves endothelial function, reduces oxidative stress | Lowers risk of nephropathy and neuropathy | Promising adjunct; long-term benefits and sex-specific dosing need validation |
Anti-CD3 antibodies
Muromonab and Teplizumab are examples of anti-CD3 monoclonal antibodies [Table 2].
Investigational clinical-stage therapies
Abatacept
Abatacept [Table 2] is a cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) immunoglobulin that consists of the Fc portion of an antibody fused to CTLA-4. CTLA-4 is an immune checkpoint protein expressed on T cells, which can interact with CD80 and CD86 on antigen-presenting cells, leading to reduced T cell activity. In a study involving patients diagnosed with T1DM for less than 100 days, continuous treatment with abatacept for over two years delayed β-cell function decline by 9.6 months. However, the overall rate of decline remained parallel to that observed in the placebo group[90]. In another study involving siblings of individuals with T1DM or participants positive for GAD antibodies, one year of abatacept treatment did not significantly delay progression from stage 1 to stage 2 or 3 T1DM, despite achieving the anticipated immunologic effects. This suggests that after the development of two or more autoantibodies, blockade of CD80 and CD86 co-stimulation may no longer be effective at that stage of disease[83]. Although further studies are required to determine its efficacy, current evidence indicates that abatacept may be most effective when administered early in the disease course [Table 3].
Mesenchymal stem cell therapy
Mesenchymal stem cells (MSCs) [Table 2] are fibroblast-like, spindle-shaped cells found in the umbilical cord, Wharton’s jelly, bone marrow, and other tissues. They can be cultured in vitro and are capable of differentiating into multiple cell lineages following implantation. MSCs have immunomodulatory properties and interact with the immune system by modulating T lymphocytes, regulatory T cells (Tregs), and TNF-α all of which are implicated in certain autoimmune diseases[91]. These properties make MSCs a promising candidate for clinical studies in autoimmune diseases such as T1DM. Several studies using MSCs derived from umbilical cord, adipose tissue, or Wharton’s jelly have demonstrated increased C-peptide levels, reduced HbA1c, and decreased daily insulin requirements[91]. Regarding safety, one study using allogeneic adipose tissue-derived stromal/stem cells combined with cholecalciferol reported adverse events, including headache, abdominal cramps, scotoma, thrombophlebitis, and mild localized reactions. However, other studies reported that MSC therapy was overall safe, with no serious adverse effects[91].
Preclinical and translational research
Treg therapy for B cell preservation
Tregs [Table 2] play a role in modulating immune responses by suppressing autoreactive cells. Dysfunction of Tregs has been implicated in the pathophysiology of T1DM[92]. Supporting this, studies have shown that patients with T1DM exhibit reduced expression of key Treg-associated mRNAs required for immunomodulation, including cytotoxic T lymphocyte-associated protein 4 (CTLA-4), interleukin-10
Vitamin D and phenolipid jambone E
Preclinical studies have suggested a role for vitamin D in the pathogenesis and management of T1DM [Table 2]. Vitamin D has been shown to protect B cells from cytokine-induced apoptosis and regulate immune responses, indicating that supplementation could potentially slow T1DM progression, as demonstrated in experimental models[94]. Moreover, patients with T1DM tend to have lower vitamin D levels than healthy individuals, and vitamin D deficiency has been associated with poorer glycemic control[94]. One study found that combining saxagliptin with vitamin D resulted in a smaller decline in the 2-hour mixed meal tolerance test C-peptide area under the curve (AUC) over 24 months compared to Saxagliptin alone. However, there was no significant difference in fasting C-peptide levels between the groups[95]. Other interventional studies have yielded conflicting results: one reported a reduction in insulin autoantibodies after 6 months of calcitriol supplementation, while another found no significant effects on C-peptide or hemoglobin A1c (HbA1c) levels[94]. Given the limited sample sizes, further studies are needed to better assess the potential of vitamin D as an adjunct therapy in T1DM [Table 3]. Jambone E (JE), a novel phenolipid compound, has shown promising antidiabetic properties. Preclinical studies have demonstrated that JE improves glucose metabolism, reduces hyperglycemia and hyperlipidemia, and enhances insulin signaling through protein kinase B (AKT) activation in both cell and mouse models. Metabolomic analyses have also identified relevant biomarkers supporting JE’s therapeutic potential in diabetes management[96]. In accordance with the findings summarized in Table 4, both clinical and preclinical studies have extensively quantified treatment outcomes in T1DM.
Summary of clinical and preclinical studies quantifying treatment outcomes in T1DM
| Therapy | Type of study | Sample size | Mean C-peptide preservation | HbA1c reduction (%) | Adverse events | Reference |
| Teplizumab | Phase 3 RCT | 328 | Preserved at 2 years | ~0.5% | Rash, lymphopenia | [89] |
| Anti-TNF (Golimumab) | Phase 2 RCT | 84 | +0.2 nmol/L over 52 weeks | ~0.3% | GI symptoms | [10] |
| Abatacept | Phase 2 RCT | 112 | N/A | ~0.2% | Mild infections | [90] |
| MSC therapy | Pilot clinical trial | 40 | +0.15 nmol/L | ~0.6% | None serious | [91] |
| Treg therapy | Phase 1 | 14 | Transient effect | N/A | Well-tolerated | [92] |
Contrasting preclinical and clinical immunological findings in T1DM
In NOD mouse models, B-cell depletion has shown marked efficacy in preventing or halting the progression of T1DM. Specifically, B-cell-deficient NOD mice do not develop spontaneous diabetes. Furthermore, disease transfer experiments demonstrate that co-transfer of diabetic splenocytes with activated B cells restores disease, whereas B cells pre-treated with anti-CD80/86 antibodies fail to induce diabetes. These findings underscore the role of B cells as APS in activating autoreactive T cells and propagating the disease[81].
In contrast, translating these findings into human clinical trials has proven challenging. Although B cells are found within human islet infiltrates and are responsible for producing disease-associated autoantibodies {e.g., anti-insulin, glutamic acid decarboxylase 65 [GAD65], insulinoma-associated antigen 2 [IA2], and zinc transporter 8 [ZnT8]}, clinical strategies targeting B cells remain limited. This is partly due to the functional heterogeneity of human B cells and the unclear pathogenic significance of autoantibodies. While anti-CD20 therapies, such as rituximab, have shown promise in delaying disease progression when administered early, they do not prevent T1DM onset and are associated with increased risks of infection and impaired immune surveillance[89].
LIMITATIONS & FUTURE DIRECTIONS
Managing T1DM in children requires maintaining stable blood glucose levels to support normal growth and development and to prevent long-term complications. Core management strategies include insulin therapy and dietary modifications, with increasing attention to the glycemic index of carbohydrates. Research shows that low-glycemic index diets can significantly improve glycemic control by reducing blood glucose fluctuations, lowering HbA1c levels, and decreasing insulin needs, likely through their impact on weight and obesity. However, adherence to dietary recommendations remains a challenge, especially due to the need to restrict certain foods, which may inadvertently lead to increased fat consumption. A well-balanced diet that limits high-glycemic index foods while maintaining adequate carbohydrate intake for growth may effectively complement insulin therapy in achieving better glycemic control in children with T1DM[97].
Despite the promise of newer diabetes technologies such as continuous subcutaneous insulin infusion (CSII) and continuous glucose monitoring (CGM), several limitations persist, involving healthcare professionals, patients, and the technology itself. Healthcare providers must be well-trained and supportive of these technologies to facilitate their adoption. However, biases in patient selection and limited provider experience with advanced tools can hinder successful implementation. From the user’s perspective, challenges include frequent sensor calibrations, infusion site changes, and interpreting real-time data, which may be overwhelming. Although structured education on flexible insulin regimens has been shown to reduce hypoglycemia, many patients still struggle with optimal glucose control. Treatment adherence remains a major obstacle, as not all patients are willing or able to consistently use these technologies. Common reasons for discontinuation include discomfort, inconvenience, and concerns about device accuracy - despite notable advances that have improved user compliance. Accuracy concerns persist, particularly due to sensor lag during rapid glucose changes, which may result in misinterpretations. Alarm fatigue is another frequent complaint, where repeated alerts can become disruptive, sometimes leading to device abandonment. Enhanced training and support systems may help mitigate these challenges and improve the effectiveness of diabetes technologies[98].
This article builds upon recent literature by providing a comprehensive synthesis of emerging insights into T1DM, specifically focusing on the roles of sex differences, the liver, and C-peptide in disease progression and management. Unlike previous reviews that primarily emphasized Treg cell-based therapies as promising but still investigational, this article expands the scope to include broader immunological mechanisms involving both T cells and B cells. It also evaluates the therapeutic potential of novel
Moreover, the article emphasizes the importance of personalized and gender-specific treatment strategies, advocating for more targeted research in these areas. By integrating the latest evidence and addressing current knowledge gaps, this article sets the stage for advancing clinical practice and improving patient outcomes in T1DM, while also recognizing the ongoing challenges and opportunities associated with
Future progress in diabetes technology, alongside improved understanding and psychological support, may help overcome existing barriers to adoption. Addressing concerns such as the fear of hypoglycemia and enhancing patient education will be crucial for optimizing both conventional and novel treatment approaches. These developments, grounded in evidence-based strategies, hold promise for improving disease management and quality of life for individuals with T1DM[98].
CONCLUSION
In conclusion, T1DM remains a complex and multifaceted disease, with ongoing advancements in understanding its pathophysiology and clinical management. Despite significant progress in areas such as disease-modifying therapies, liver involvement, and the emerging relevance of C-peptide, challenges persist in achieving optimal disease control and preventing long-term complications. The exploration of sex-based differences in disease presentation, along with the development of novel therapeutic strategies, presents new avenues for improving patient outcomes. Future research should continue to address these gaps, particularly in the context of personalized and gender-specific treatments. By building upon these insights, there is hope for advancing the management and potential modification of T1DM, ultimately improving the quality of life for individuals living with this chronic condition.
DECLARATIONS
Acknowledgments
The authors would like to acknowledge the Faculty of Medicine and Medical Sciences at University of Balamand.
Authors’ contributions
Conception, organization: Joumaa JP, Attieh P, Raffoul A, Ghadieh HE,
Writing of the first draft: Joumaa JP, Chatrieh E, Sarkis C
Review/editing: Joumaa JP, Attieh P, Harb F, Azar S, Ghadieh HE
Conception and design: Ghadieh HE
All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiol Behav. 2018;187:20-3.
2. Memaj P, Jornayvaz FR. Non-alcoholic fatty liver disease in type 1 diabetes: Prevalence and pathophysiology. Front Endocrinol. 2022;13:1031633.
3. Vries M, Westerink J, Kaasjager KHAH, de Valk HW. Prevalence of nonalcoholic fatty liver disease (NAFLD) in patients with type 1 diabetes mellitus: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2020;105:3842-53.
4. Shi Y, Wang Q, Sun Y, et al. The prevalence of lean/nonobese nonalcoholic fatty liver disease: a systematic review and meta-analysis. J Clin Gastroenterol. 2020;54:378-87.
5. Ryk A, Łosiewicz A, Michalak A, Fendler W. Biological activity of c-peptide in microvascular complications of type 1 diabetes-time for translational studies or back to the basics? Int J Mol Sci. 2020;21:9723.
6. Wahren J, Larsson C. C-peptide: new findings and therapeutic possibilities. Diabetes Res Clin Pract. 2015;107:309-19.
7. Mastrandrea L, Yu J, Behrens T, et al. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32:1244-9.
8. Quattrin T, Haller MJ, Steck AK, et al; T1GER Study Investigators. Golimumab and beta-cell function in youth with new-onset type 1 diabetes. N Engl J Med. 2020;383:2007-17.
9. Herold KC, Delong T, Perdigoto AL, Biru N, Brusko TM, Walker LSK. The immunology of type 1 diabetes. Nat Rev Immunol. 2024;24:435-51.
10. Bazile C, Abdel Malik MM, Ackeifi C, et al. TNF-α inhibitors for type 1 diabetes: exploring the path to a pivotal clinical trial. Front Immunol. 2024;15:1470677.
11. Vries SAG, Verheugt CL, Mul D, Nieuwdorp M, Sas TCJ. Do sex differences in paediatric type 1 diabetes care exist? Diabetologia. 2023;66:618-30.
12. Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481-97.
13. Agarwal S, Kanapka LG, Raymond JK, et al. Racial-ethnic inequity in young adults with type 1 diabetes. J Clin Endocrinol Metab. 2020;105:e2960-9.
14. Shepard BD. Sex differences in diabetes and kidney disease: mechanisms and consequences. Am J Physiol Renal Physiol. 2019;317:F456-62.
15. Giandalia A, Giuffrida AE, Gembillo G, et al. Gender differences in diabetic kidney disease: focus on hormonal, genetic and clinical factors. Int J Mol Sci. 2021;22:5808.
16. Ohkuma T, Komorita Y, Peters SAE, Woodward M. Diabetes as a risk factor for heart failure in women and men: a systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia. 2019;62:1550-60.
17. Chase-Vilchez AZ, Chan IHY, Peters SAE, Woodward M. Diabetes as a risk factor for incident peripheral arterial disease in women compared to men: a systematic review and meta-analysis. Cardiovasc Diabetol. 2020;19:151.
18. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542-51.
19. Wang Y, O'Neil A, Jiao Y, et al. Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: a systematic review and meta-analysis of 5,162,654 participants. BMC Med. 2019;17:136.
20. Wei G, Zhu JJ, Shen FJ, et al. Heterogeneous metabolic changes of brown and white adipose tissues are associated with metabolic adaptations in periparturient mice. J Mol Endocrinol. 2024;73:e240012.
21. Frias JP, Macaraeg GB, Ofrecio J, Yu JG, Olefsky JM, Kruszynska YT. Decreased susceptibility to fatty acid-induced peripheral tissue insulin resistance in women. Diabetes. 2001;50:1344-50.
22. Nuutila P, Knuuti MJ, Mäki M, et al. Gender and insulin sensitivity in the heart and in skeletal muscles: studies using positron emission tomography. Diabetes. 1995;44:31-6.
23. Brener A, Hamama S, Interator H, et al. Sex differences in body composition in youth with type 1 diabetes and its predictive value in cardiovascular disease risk assessment. Diabetes Metab Res Rev. 2023;39:e3584.
24. Schweiger D, Battelino T, Groselj U. Sex-related differences in cardiovascular disease risk profile in children and adolescents with type 1 diabetes. Int J Mol Sci. 2021;22:10192.
25. Braffett BH, Bebu I, El Ghormli L, et al; DCCT/EDIC Research Group. Cardiometabolic risk factors and incident cardiovascular disease events in women vs men with type 1 diabetes. JAMA Netw Open. 2022;5:e2230710.
26. Nyström L, Dahlquist G, Ostman J, et al. Risk of developing insulin-dependent diabetes mellitus (IDDM) before 35 years of age: indications of climatological determinants for age at onset. Int J Epidemiol. 1992;21:352-8.
27. Blohmé G, Nyström L, Arnqvist HJ, et al. Male predominance of type 1 (insulin-dependent) diabetes mellitus in young adults: results from a 5-year prospective nationwide study of the 15-34-year age group in Sweden. Diabetologia. 1992;35:56-62.
28. Laing SP, Swerdlow AJ, Slater SD, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia. 2003;46:760-5.
29. Harjutsalo V, Maric-Bilkan C, Forsblom C, Groop PH. Impact of sex and age at onset of diabetes on mortality from ischemic heart disease in patients with type 1 diabetes. Diabetes Care. 2014;37:144-8.
30. Bisson A, Bodin A, Fauchier G, et al. Sex, age, type of diabetes and incidence of atrial fibrillation in patients with diabetes mellitus: a nationwide analysis. Cardiovasc Diabetol. 2021;20:24.
31. Tas E, Vu BK, Mendizabal B, Libman I, Muzumdar R. Relationship between liver and cardiometabolic health in type 1 diabetes. Front Endocrinol. 2024;15:1505430.
32. Rakusanova S, Cajka T. Metabolomics and lipidomics for studying metabolic syndrome: insights into cardiovascular diseases, type 1 & 2 diabetes, and metabolic dysfunction-associated steatotic liver disease. Physiol Res. 2024;73:S165-83.
33. Boulos M, Mousa RS, Jeries N, et al. Hidden in the fat: unpacking the metabolic tango between metabolic dysfunction-associated steatotic liver disease and metabolic syndrome. Int J Mol Sci. 2025;26:3448.
34. Bourganou MV, Chondrogianni ME, Kyrou I, et al. Unraveling metabolic dysfunction-associated steatotic liver disease through the use of omics technologies. Int J Mol Sci. 2025;26:1589.
35. Wang JS, Yen FS, Lin KD, Shin SJ, Hsu YH, Hsu CC; Diabetes Kidney Disease Research Committee of the Diabetes Association of the Republic of China (Taiwan). Epidemiological characteristics of diabetic kidney disease in Taiwan. J Diabetes Investig. 2021;12:2112-23.
36. Lord S, Greenbaum CJ. Insulin is necessary but not sufficient: changing the therapeutic paradigm in type 1 diabetes. F1000Res. 2020;9:827.
38. Im SS, Kang SY, Kim SY, et al. Glucose-stimulated upregulation of GLUT2 gene is mediated by sterol response element-binding protein-1c in the hepatocytes. Diabetes. 2005;54:1684-91.
39. Thorens B, Flier JS, Lodish HF, Kahn BB. Differential regulation of two glucose transporters in rat liver by fasting and refeeding and by diabetes and insulin treatment. Diabetes. 1990;39:712-9.
40. Gastaldelli A, Cusi K. From NASH to diabetes and from diabetes to NASH: mechanisms and treatment options. JHEP Rep. 2019;1:312-28.
41. Manco M. Insulin resistance and NAFLD: a dangerous liaison beyond the genetics. Children. 2017;4:74.
42. Petta S, Gastaldelli A, Rebelos E, et al. Pathophysiology of non alcoholic fatty liver disease. Int J Mol Sci. 2016;17:2082.
43. Mertens J, De Block C, Spinhoven M, Driessen A, Francque SM, Kwanten WJ. Hepatopathy associated with type 1 diabetes: distinguishing non-alcoholic fatty liver disease from glycogenic hepatopathy. Front Pharmacol. 2021;12:768576.
44. Mohamed J, Nazratun Nafizah AH, Zariyantey AH, Budin SB. Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation. Sultan Qaboos Univ Med J. 2016;16:e132-41.
45. Rafaqat S, Sattar A, Khalid A, Rafaqat S. Role of liver parameters in diabetes mellitus - a narrative review. Endocr Regul. 2023;57:200-20.
46. West J, Brousil J, Gazis A, et al. Elevated serum alanine transaminase in patients with type 1 or type 2 diabetes mellitus. QJM. 2006;99:871-6.
47. Huo G, Gao Y. Type 1 diabetes and combined acute and chronic complications are associated with risk of progression of liver fibrosis: a Mendelian randomization study. Front Endocrinol. 2024;15:1302611.
48. Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376:1407-18.
49. Donaghue K, Jeanne Wong SL. Traditional cardiovascular risk factors in adolescents with type 1 diabetes mellitus. Curr Diabetes Rev. 2017;13:533-43.
50. Della Pepa G, Lupoli R, Masulli M, et al. Blood glucose control and metabolic dysfunction-associated steatotic liver disease in people with type 1 diabetes. J Endocrinol Invest. 2024;47:2371-8.
51. Caldara R, Tomajer V, Monti P, et al. Allo beta cell transplantation: specific features, unanswered questions, and immunological challenge. Front Immunol. 2023;14:1323439.
52. Zhou A, Webb G, Zhu X, Steiner DF. Proteolytic processing in the secretory pathway. J Biol Chem. 1999;274:20745-8.
53. Chima RS, Maltese G, Lamontagne T, et al. C-peptide ameliorates kidney injury following hemorrhagic shock. Shock. 2011;35:524-9.
54. Luppi P, Cifarelli V, Tse H, Piganelli J, Trucco M. Human C-peptide antagonises high glucose-induced endothelial dysfunction through the nuclear factor-κB pathway. Diabetologia. 2008;51:1534-43.
55. Krishna SV, Kota SK, Modi KD. Glycemic variability: clinical implications. Indian J Endocrinol Metab. 2013;17:611-9.
56. Ido Y, Vindigni A, Chang K, et al. Prevention of vascular and neural dysfunction in diabetic rats by C-peptide. Science. 1997;277:563-6.
57. Sima AA, Zhang W, Sugimoto K, et al. C-peptide prevents and improves chronic Type I diabetic polyneuropathy in the BB/Wor rat. Diabetologia. 2001;44:889-97.
58. Redondo MJ, Geyer S, Steck AK, et al; Type 1 Diabetes TrialNet Study Group. A type 1 diabetes genetic risk score predicts progression of islet autoimmunity and development of type 1 diabetes in individuals at risk. Diabetes Care. 2018;41:1887-94.
59. Redondo MJ, Sosenko J, Libman I, et al. Single islet autoantibody at diagnosis of clinical type 1 diabetes is associated with older age and insulin resistance. J Clin Endocrinol Metab. 2020;105:1629-40.
60. Leslie RD, Evans-Molina C, Freund-Brown J, et al. Adult-onset type 1 diabetes: current understanding and challenges. Diabetes Care. 2021;44:2449-56.
61. Jamiołkowska-Sztabkowska M, Głowińska-Olszewska B, Bossowski A. C-peptide and residual β-cell function in pediatric diabetes - state of the art. Pediatr Endocrinol Diabetes Metab. 2021;27:123-33.
62. Wahren J. C-peptide and the pathophysiology of microvascular complications of diabetes. J Intern Med. 2017;281:3-6.
63. Luppi P, Kallas Å, Wahren J. Can C-peptide mediated anti-inflammatory effects retard the development of microvascular complications of type 1 diabetes? Diabetes Metab Res Rev. 2013;29:357-62.
64. Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013;30:803-17.
65. Wahren J, Kallas A, Sima AA. The clinical potential of C-peptide replacement in type 1 diabetes. Diabetes. 2012;61:761-72.
66. Washburn RL, Mueller K, Kaur G, et al. C-peptide as a therapy for type 1 diabetes mellitus. Biomedicines. 2021;9:270.
67. Marques RG, Fontaine MJ, Rogers J. C-peptide: much more than a byproduct of insulin biosynthesis. Pancreas. 2004;29:231-8.
68. Chen J, Huang Y, Liu C, Chi J, Wang Y, Xu L. The role of C-peptide in diabetes and its complications: an updated review. Front Endocrinol. 2023;14:1256093.
69. Alam S, Khan SJ, Lee CYF, Zaidi SAT, Murtaza SF. Type 1 diabetes mellitus management and islet cell therapy: a new chapter in patient care. Cureus. 2023;15:e46912.
70. Bruemmer D. C-Peptide in insulin resistance and vascular complications: teaching an old dog new tricks. Circ Res. 2006;99:1149-51.
71. Walcher D, Babiak C, Poletek P, et al. C-Peptide induces vascular smooth muscle cell proliferation: involvement of SRC-kinase, phosphatidylinositol 3-kinase, and extracellular signal-regulated kinase 1/2. Circ Res. 2006;99:1181-7.
72. Maddaloni E, Bolli GB, Frier BM, et al. C-peptide determination in the diagnosis of type of diabetes and its management: a clinical perspective. Diabetes Obes Metab. 2022;24:1912-26.
73. Otto-Buczkowska E. The clinical utility of C-peptide measurement in diabetology. Pediatr Endocrinol Diabetes Metab. 2015;20:63-8.
74. Mathieu C, Wiedeman A, Cerosaletti K, et al; AG019-T1D-101 Trial Investigators. A first-in-human, open-label Phase 1b and a randomised, double-blind Phase 2a clinical trial in recent-onset type 1 diabetes with AG019 as monotherapy and in combination with teplizumab. Diabetologia. 2024;67:27-41.
75. Herold KC, Gitelman SE, Willi SM, et al. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia. 2013;56:391-400.
76. Perdigoto AL, Preston-Hurlburt P, Clark P, et al; Immune Tolerance Network. Treatment of type 1 diabetes with teplizumab: clinical and immunological follow-up after 7 years from diagnosis. Diabetologia. 2019;62:655-64.
77. Taylor PN, Collins KS, Lam A, et al; Trial Outcome Markers Initiative collaboration. C-peptide and metabolic outcomes in trials of disease modifying therapy in new-onset type 1 diabetes: an individual participant meta-analysis. Lancet Diabetes Endocrinol. 2023;11:915-25.
78. Dwyer AJ, Shaheen ZR, Fife BT. Antigen-specific T cell responses in autoimmune diabetes. Front Immunol. 2024;15:1440045.
79. Durinovic-Belló I. Autoimmune diabetes: the role of T cells, MHC molecules and autoantigens. Autoimmunity. 1998;27:159-77.
80. Xie Z, Chang C, Zhou Z. Molecular mechanisms in autoimmune type 1 diabetes: a critical review. Clin Rev Allergy Immunol. 2014;47:174-92.
81. Khosravi-Maharlooei M, Madley R, Borsotti C, et al. Modeling human T1D-associated autoimmune processes. Mol Metab. 2022;56:101417.
82. Yue M, He X, Min X, et al. The role of islet autoantigen-specific T cells in the onset and treatment of type 1 diabetes mellitus. Front Immunol. 2024;15:1462384.
83. Russell WE, Moore DJ, Herold KC. Response to comment on Russell et al. Abatacept for Delay of Type 1 Diabetes Progression in Stage 1 Relatives at Risk: A Randomized, Double-Masked, Controlled Trial. Diabetes Care 2023;46:1005-1013. Diabetes Care. 2023;46:e210-1.
84. Blagov AV, Summerhill VI, Sukhorukov VN, Popov MA, Grechko AV, Orekhov AN. Type 1 diabetes mellitus: inflammation, mitophagy, and mitochondrial function. Mitochondrion. 2023;72:11-21.
85. Basaran E, Aktas G. Waist-to-height ratio as a novel marker of metabolic syndrome in patients with type 2 diabetes mellitus. Explor Endocr Metab Dis. 2025;2:101421.
86. Kosekli MA, Aktas G. The systemic immune inflammation index is a reliable and novel risk factor for metabolic dysfunction-associated fatty liver disease. Curr Med Res Opin. 2025;41:247-51.
87. Czajkowska JB, Shutty B, Zito S. Development of low blood glucose readings in nine non-diabetic patients treated with tumor necrosis factor-alpha inhibitors: a case series. J Med Case Rep. 2012;6:5.
88. Thakkar S, Chopra A, Nagendra L, Kalra S, Bhattacharya S. Teplizumab in type 1 diabetes mellitus: an updated review. touchREV Endocrinol. 2023;19:22-30.
89. Herold KC, Gitelman SE, Gottlieb PA, Knecht LA, Raymond R, Ramos EL. Teplizumab: a disease-modifying therapy for type 1 diabetes that preserves β-cell function. Diabetes Care. 2023;46:1848-56.
90. Orban T, Bundy B, Becker DJ, et al; Type 1 Diabetes TrialNet Abatacept Study Group. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412-9.
91. Jasim SA, Yumashev AV, Abdelbasset WK, et al. Shining the light on clinical application of mesenchymal stem cell therapy in autoimmune diseases. Stem Cell Res Ther. 2022;13:101.
92. Loretelli C, Assi E, Seelam AJ, Ben Nasr M, Fiorina P. Cell therapy for type 1 diabetes. Expert Opin Biol Ther. 2020;20:887-97.
93. Wang Z, Xu J, Mo L, et al. The application potential of the regulation of tregs function by irisin in the prevention and treatment of immune-related diseases. Drug Des Devel Ther. 2024;18:3005-23.
94. Bellan M, Andreoli L, Mele C, et al. Pathophysiological role and therapeutic implications of vitamin d in autoimmunity: focus on chronic autoimmune diseases. Nutrients. 2020;12:789.
95. Yan X, Li X, Liu B, et al. Combination therapy with saxagliptin and vitamin D for the preservation of β-cell function in adult-onset type 1 diabetes: a multi-center, randomized, controlled trial. Signal Transduct Target Ther. 2023;8:158.
96. Wang G, Xu J, Ma H, et al. Phenolipid JE improves metabolic profile and inhibits gluconeogenesis via modulating AKT-mediated insulin signaling in STZ-induced diabetic mice. Pharmacol Res. 2023;187:106569.
97. Quarta A, Guarino M, Tripodi R, Giannini C, Chiarelli F, Blasetti A. Diet and glycemic index in children with type 1 diabetes. Nutrients. 2023;15:3507.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].