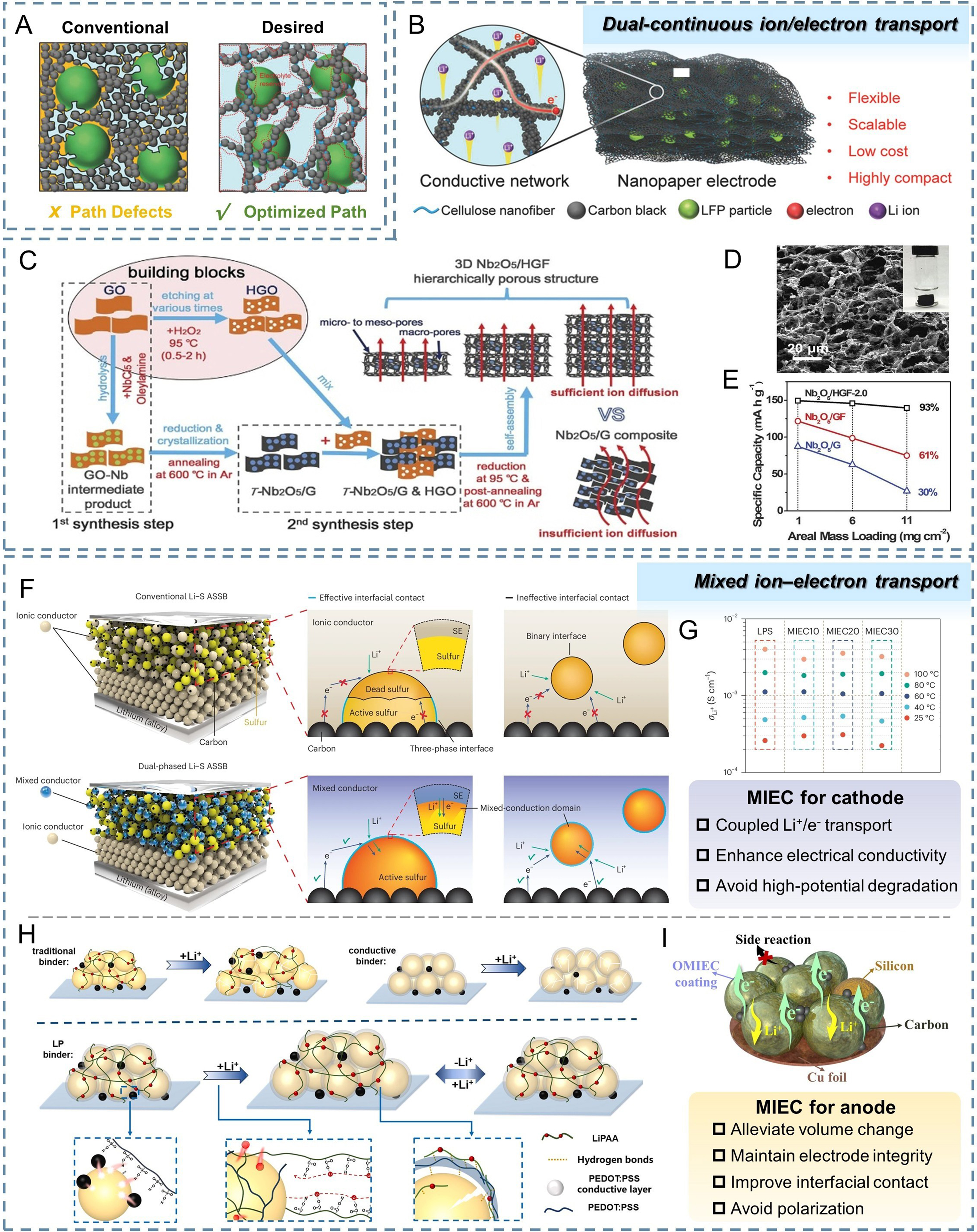
fig2
Figure 2. High-performance thick electrodes for constructing ion-electron cooperative transport networks. (A) Schematic of ion-electron transport pathways in conventional electrodes versus desired electrodes; (B) Electrostatic self-assembly enabling spatial decoupling of ion/electron transport pathways. (A and B) is quoted with permission from Ref.[26], Copyright © 2018 Wiley; (C) Two-step synthesis yielding an Nb2O5/HGF composite architecture with independent three-dimensional hierarchical pores; (D) SEM image of the three-dimensional hierarchical porous structure; (E) Comparison of capacity retention rates for electrodes with different loading levels. (C-E) are quoted with permission from Ref.[27], Copyright © 2017 American Association for the Advancement of Science; (F) MIEC introduced a sulfur cathode to establish a spatially coupled ion-electron transport network; (G) The ion conductivity measurements at different temperatures. (F and G) are quoted with permission from Ref.[30], Copyright © 2015 Springer Nature; (H) A trifunctional MIEC serves as a conductive binder for silicon anodes. This figure is quoted with permission from Ref.[31], Copyright © 2024 ELSEVIER; (I) MIEC is coated onto the surface of active particles. This figure is quoted with permission from Ref.[32], Copyright © 2026 ELSEVIER. HGF: Holey graphene framework; SEM: scanning electron microscope; MIEC: mixed ion-electron conductor; LFP: LiFePO4; Go-Nb: graphene oxide Nb2O5; HGO: holey graphene oxide; GF: graphene framework; G: graphene; ASSB: all-solid-state batteries; SE: solid electrolytes; LPS: Li2S·25P2S5; LP: lignin polymer; LiPAA: lithiated polyacrylic acid; PEDOT: poly(3,4-ethylenedioxythiophene); PSS: poly(styrenesulfonate); OMIEC: organic mixed ionic/electronic conductors.