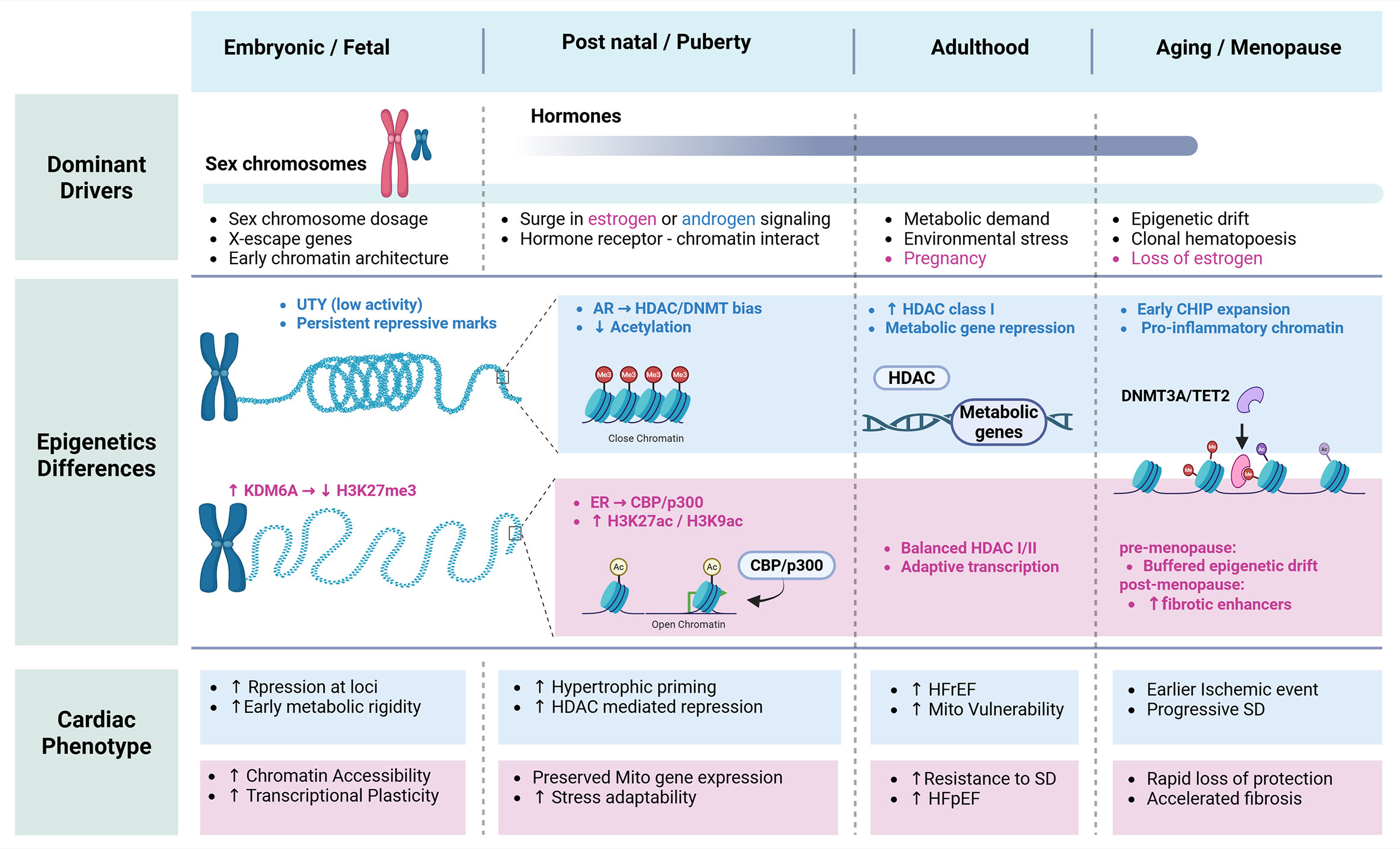
fig3
Figure 3. Sex-specific epigenetic regulation of cardiac phenotype across the lifespan.Schematic showing how sex chromosomes, hormones, and aging shape epigenetic regulation and cardiac phenotype from embryonic development through adulthood and menopause. Early sex chromosome dosage establishes divergent chromatin states, with males exhibiting greater repressive marks and females showing increased chromatin accessibility. Pubertal hormone signaling further biases epigenetic regulation, favoring HDAC/DNMT-mediated repression in males and estrogen-dependent histone acetylation and transcriptional plasticity in females. In adulthood and aging, these sex-specific epigenetic trajectories contribute to differential cardiac remodeling, heart failure susceptibility, ischemic risk, and fibrosis. UTY: ubiquitously transcribed tetratricopeptide repeat containing: Y-linked; KDM6A (UTX): lysine demethylase 6A; AR: androgen receptor; ER: estrogen receptor; CBP: CREB-binding protein; p300: E1A-associated protein p300; HDAC: histone deacetylase; DNMT: DNA methyltransferase; TET2: ten-eleven translocation 2; SD: systolic dysfunction; CHIP: clonal hematopoiesis of indeterminate potential. Created in BioRender. Rouzbehani, O. (2026) https://BioRender.com/1ihtyg3.