Microneedle-mediated therapies in hair loss: applications, mechanisms, and future directions
Abstract
Microneedles, as minimally invasive tools for percutaneous delivery, have undergone significant technological advancements over the past decade, greatly expanding their application in the treatment of hair loss. Traditional microneedle devices promote hair regrowth by stimulating vascularization and the release of growth factors through controlled skin disruption. In addition, microneedle-mediated drug delivery enhances local drug penetration and absorption, thereby regulating the hair growth cycle more effectively. During hair transplantation procedures, microneedle-assisted techniques can notably improve follicle survival rates due to their precision and advanced design. For individuals with baldness, tissue-engineered hair follicle regeneration offers an innovative and effective strategy to increase the total number of hair follicles. However, these engineered follicles often face challenges, such as difficulty in emerging through the skin and growing in excessively dense or disorganized patterns. Integrating microneedle arrays with tissue engineering approaches may help address these issues by enabling better control over the direction and density of regenerated hair. This article reviews the historical development of microneedles, examines their current applications in hair loss therapy, and discusses ongoing challenges in the field. The goal is to explore the therapeutic potential of microneedles in hair regeneration and to offer insights that may guide future treatment strategies.
Keywords
INTRODUCTION
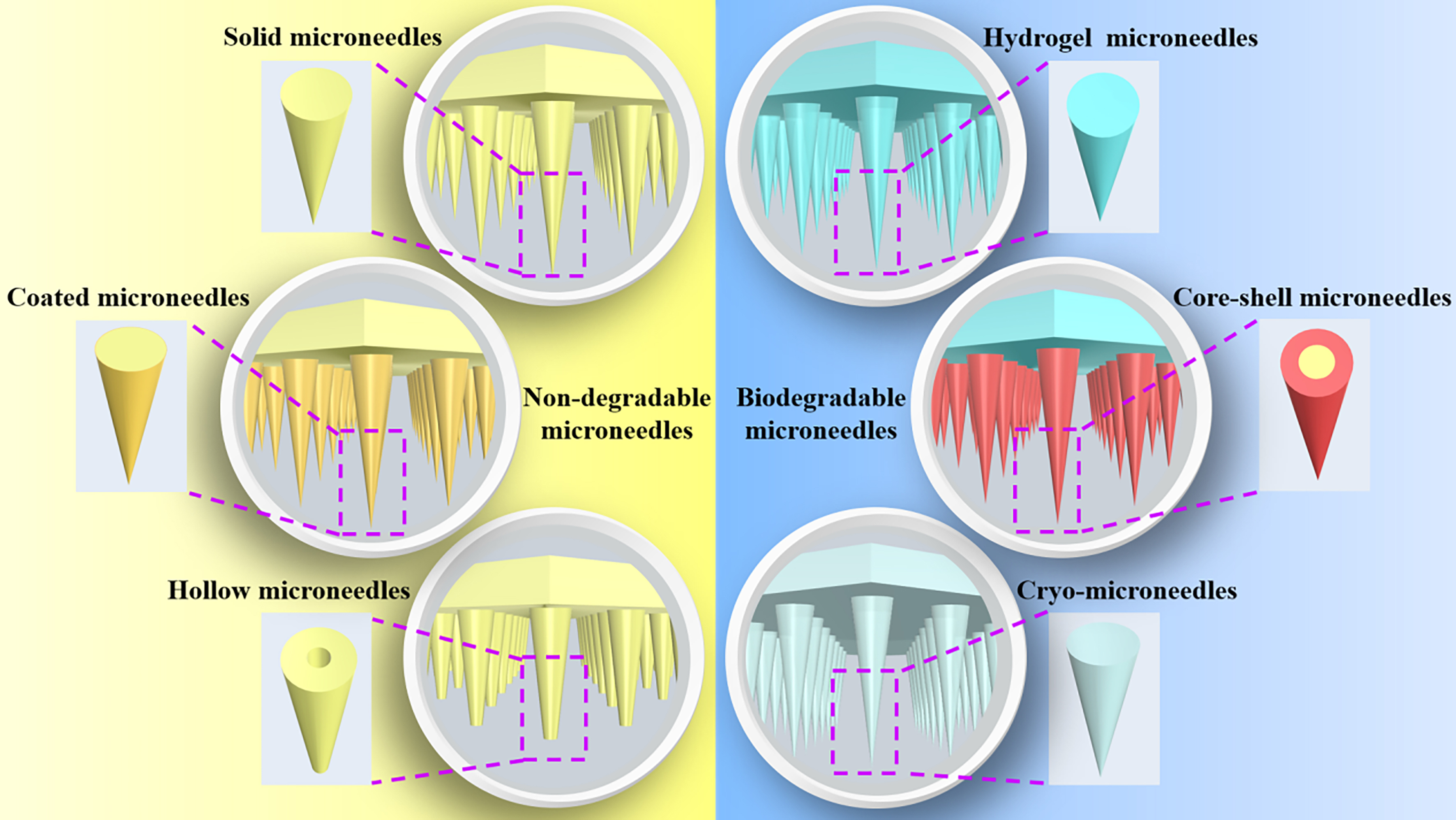
Microneedle (MN) delivery has been a significant area of research over the past few decades. This method effectively overcomes the skin’s natural barrier, enabling the transport of molecules through the skin in a minimally invasive manner that avoids discomfort for the patient[1,2]. Designed in conical or pyramidal shapes, MNs are engineered to penetrate the dermis smoothly. They can be fabricated as single units or in arrays, with adjustable diameters and lengths tailored to the size of the drug molecules and the location of the target tissues - typically ranging from several hundred microns to one millimeter. MNs enable the rapid delivery of drugs, genes, and vaccines into the dermal and subcutaneous layers, allowing for efficient targeting of specific tissues. This therapy is generally painless and considered non-invasive, garnering strong patient endorsement due to the avoidance of nerve-ending penetration[3,4]. Currently, MNs are extensively used in the treatment of skin and subcutaneous disorders, particularly in the field of cosmetology[5]. They can deliver therapeutic agents to specific skin areas or enable systemic drug delivery via transdermal absorption. MNs are broadly classified into two categories based on degradability: non-degradable and biodegradable. Non-degradable types include solid, coated, and hollow MNs, while biodegradable varieties encompass hydrogel, core-shell, and cryo-microneedles (cryoMNs) [Figure 1]. Additionally, various MN-based devices, such as roller, electric, seal, and Radiofrequency MNs (FRM), are used across a range of applications.
Figure 1. Classification of microneedles. Microneedles are divided into two main categories based on their degradability: non-degradable microneedles and biodegradable microneedles. Non-degradable microneedles include solid, coated, and hollow microneedles. Biodegradable microneedles include hydrogel microneedles, core-shell microneedles, and cryoMNs. cryoMNs: Cryo-microneedles.
Alopecia, a common condition, is increasingly affecting individuals at younger ages. It not only alters physical appearance but also imposes a substantial psychological burden[6]. Current treatment options for alopecia include medications, laser therapy, injection therapy, and surgical interventions. However, traditional drug treatments such as minoxidil (MXD) and finasteride (FNS) are often associated with low absorption and undesirable side effects; hair transplant surgery is invasive, costly, and limited in scope; and laser therapy and platelet-rich plasma (PRP) injections vary in effectiveness among individuals and are expensive. MN therapy offers a safer and more effective non-surgical alternative for treating hair loss. By creating micro-injuries in scalp tissue, MN therapy stimulates angiogenesis and the release of growth factors, promoting hair growth and regulating the hair cycle through enhanced Wnt protein expression[7]. Moreover, MNs improve drug absorption and targeted delivery to scalp tissues, thereby enhancing the effectiveness of hair loss treatments[7-9]. MNs are also used as auxiliary tools in hair transplantation. The use of MN pens has significantly enhanced the success of hair transplant procedures[10]. In traditional hair transplantation, follicles are extracted from the occipital area and implanted into bald areas; however, this technique does not increase the total number of follicles. Hair follicle tissue engineering holds promise for expanding the number of hair follicles[11,12], but efficient delivery of these expanded follicle cells to bald areas remains a significant challenge. This paper reviews the current applications of MNs in hair loss treatment and explores their potential future roles in hair regeneration, aiming to provide new insights and directions for therapeutic innovation in this field.
ORIGIN AND DEVELOPMENT OF MNS ACROSS DIVERSE APPLICATION FIELDS
Proposal and construction of MN technology
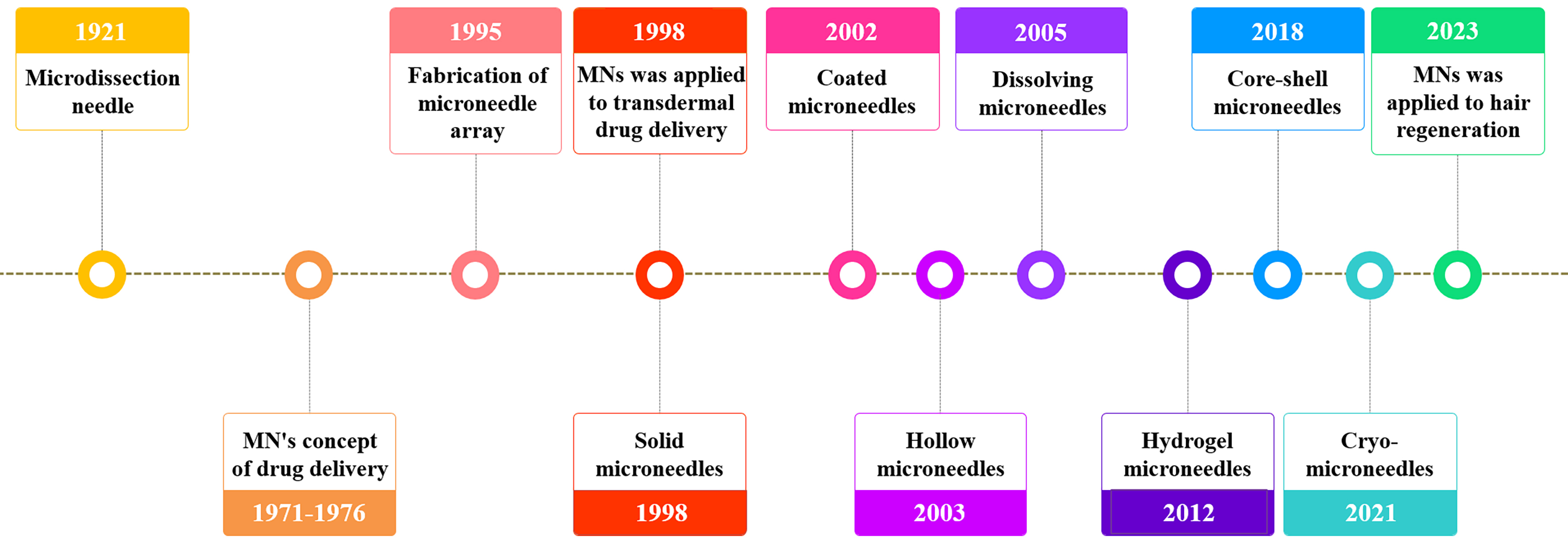
The concept of MNs dates back to 1921, when Chambers introduced the microdissection needle to extract germinal vesicles from starfish eggs[13]. The idea of using MNs for drug delivery was first reported in a U.S. patent filed on May 17, 1971, and granted on June 22, 1976[14]. In this patent, Gerstel and Place described a drug delivery device comprising a MN array designed to penetrate the skin for local or systemic administration. However, the concept could not be implemented at the time due to technological limitations. With the development of microelectromechanical systems (MEMS) and the emergence of high-precision semiconductor and microelectronic devices in 1995, MN arrays were first fabricated using etching techniques on silicon wafers[15]. In 1998, Henry et al. applied MNs to transdermal drug delivery, significantly improving skin permeability to the model compound calcein[16]. Standard microfabrication techniques were employed to prepare micron-scale silicon MNs that, upon skin penetration, created transport channels through the stratum corneum, allowing drug molecules to reach underlying capillaries for systemic absorption. Experiments on healthy human skin demonstrated that MNs enhanced the transdermal diffusion of calcein by nearly four orders of magnitude without causing pain or discomfort. Since then, MN design and materials have continuously evolved. Recent advancements have enabled the development of drug-loaded MNs with exceptional and consistent chemical properties. Major breakthroughs in MN technology over the past century are illustrated in Figure 2 and will be discussed in detail in subsequent sections.
Figure 2. Historical timeline of microneedle development. Chambers R introduced the microdissection needle in 1921[13]. Gerstel and Place first proposed the concept of “Microneedle drug loading” in 1971-1976[14]. Hashmi et al. fabricated microneedle arrays in 1995[15]. Henry et al. applied solid microneedles for transdermal drug delivery in 1998[16]. Matriano et al. reported the first coated microneedle in 2002[17]. McAllister et al. developed the first hollow microneedle in 2003[18]. Miyano et al. created the first dissolving microneedle in 2005[19]. Kim et al. first introduced the gel microneedle in 2012[20]. Wang et al. proposed the concept of core-shell microneedles in 2018[21]. Chang et al. developed cryoMNs for delivering living cells in 2021[22]. Zheng et al. applied microneedles to hair regeneration in 2023[23]. MN: Microneedle; cryoMNs: cryo-microneedles.
Classification and characteristics of MNs
Solid MNs are typically made from non-biodegradable materials such as silicon[24], metal[25,26] and ceramics[27]. These MNs possess advantages such as a rigid texture and strong puncture capability. However, they are not effective drug carriers; only a small amount of the drug applied to the skin surface penetrates before the microchannels created by the needles close naturally. To address this issue, MNs capable of delivering drugs were developed. In 2002, Matriano et al. reported the first coated MN, which involved applying a model protein antigen, ovalbumin (OVA), to the surface of titanium MNs to enhance the antibody response[17]. Since then, coated MNs have evolved into a more advanced platform for precise transdermal drug delivery. For example, in 2004, Cormier et al. coated a solid layer of desmopressin onto MN tips, achieving effective vasopressin absorption through the skin of hairless guinea pigs and significantly improving bioavailability[26]. Despite these advances, coated MNs still face limitations. The amount of drug they can carry is low, and the coating may dull the MN tip. Additionally, friction during application may cause some of the coating to remain on the outer skin layers, preventing the drug from reaching deeper dermal tissues and reducing its efficacy. Furthermore, individual variability in skin pore formation can lead to inconsistencies in dosage and errors in pharmacokinetic and pharmacodynamic evaluations.
To increase drug loading capacity, McAllister et al. developed the first solid hollow MN in 2003 using materials such as silicon, metal, polymers, and glass[18]. In 2005, Wang et al. synthesized a hollow glass MN that was used in clinical settings to extract dermal interstitial fluid (ISF) for blood glucose monitoring[28]. Hollow MNs allow for rapid drug delivery, either through internal drug reservoirs or by external pressure application[29]. Their main advantage lies in their capacity to carry larger drug volumes and enable controlled release for precise dosing. However, tissue may block the needle’s opening during insertion, increasing resistance to drug flow. To address this, the opening can be positioned on the side of the needle tip, and partial insertion can help facilitate infusion[30].
While solid MNs are mechanically robust and can penetrate the skin easily, they also risk breaking and remaining in the body, which poses safety concerns. To improve safety, Miyano et al. created the first dissolving MN (DMN) in 2005 by combining maltose with a drug, which dissolved upon insertion, releasing ascorbic acid in the epidermis and dermis and safely degrading in the skin[19]. Biodegradable materials greatly enhance the biosafety of MNs and have become a focus of extensive research. Common polymers used include polylactic acid[31], hyaluronic acid (HA)[32,33] and polycarbonate[34]. DMNs eliminate the need for removal, thereby improving patient compliance and reducing the risk of cross-infection. Key fabrication technologies, such as casting, hot pressing, injection molding, and micro-plastic molding, allow for reduced production costs compared to other MN types. The primary challenge lies in developing materials that are both biodegradable and capable of penetrating the skin effectively.
Hydrogel-forming MNs, a subtype of DMNs, are made from hydrogel-forming polymer matrices. These MNs swell upon absorbing water, creating channels through which the drug is delivered[35]. In 2012,
However, due to the limited elasticity of the skin and the low mechanical strength of hydrogels, these MNs often fail to fully penetrate or dissolve in the skin. In 2018, researchers developed a novel degradable MN with a core-shell structure[37] that enhanced drug delivery while maintaining sufficient puncture strength. These MNs consist of a drug-loaded HA shell and a drug-free polyvinyl alcohol (PVA) backing layer. The rigid PVA backing provides the mechanical strength needed for skin penetration, while the HA shell ensures effective drug release. In the same year, Wang et al. proposed the concept of core-shell MNs[21], preparing a biodegradable cross-linked PVA gel system for insulin delivery to regulate blood glucose levels.
While MN-based drug delivery systems have matured, delivering viable living cells remains challenging. In 2021, Chang et al. introduced the concept of cryoMNs[22], in which OVA-pulsed dendritic cells were encapsulated in a low-temperature medium and formed into MNs through gradient freezing for cancer immunotherapy. In 2023, Zheng et al. embedded hair follicle organoids in gelatin methacryloyl (GelMA) hydrogel to produce cryoMNs[23]. Fourteen days after application to the dorsal skin of nude mice, hair regeneration was observed.
MN APPLICATIONS IN HAIR LOSS MANAGEMENT
Microneedling offers several advantages in the treatment of hair loss. First, it significantly improves drug penetration by creating microscopic channels in the skin, allowing therapeutic agents to reach the hair follicles more effectively and thereby improving treatment outcomes. Second, microneedling stimulates the cells surrounding the hair follicles, promoting hair growth, improving the follicle microenvironment, and reducing inflammation and oxidative stress. Additionally, when combined with 5% MXD, growth factor solutions and/or PRP, microneedling demonstrates significantly greater efficacy compared to the use of these agents alone. Moreover, microneedling is easy to administer, safe, and applicable to various types of hair loss, including androgenic alopecia (AGA) and alopecia areata (AA), with proven effectiveness across different genders and severities of hair loss.
Microneedling devices for hair growth: origins and development
Over the past decade, microneedling devices have undergone continuous development for hair restoration purposes. In 2010, You et al. used polymer roller MNs to improve the skin permeability of L-ascorbic acid and promote hair growth in rats[38]. To date, roller MNs remain the most widely used microneedling device. These comprise a handle attached to a roller embedded with several rows of MNs, typically 0.5~3 mm in length. Rolling the device across the skin creates micro-channels that stimulate cell proliferation. In 2013, Dhurat et al. conducted the first clinical study using roller MNs in combination with 5% MXD to treat alopecia in humans. The study demonstrated a significant increase in hair count among patients with AGA[39]. In this protocol, 1.5 mm roller MNs were applied longitudinally, vertically, and diagonally across the scalp until mild erythema appeared. That same year, a South Korean study first introduced the use of growth factors combined with roller microneedling to treat female pattern hair loss, also reporting significant improvements in hair density[40]. Electric MNs function by mechanically disrupting the skin’s stratum corneum to create temporary channels that facilitate the penetration of drug molecules into the epidermis. These devices typically consist of a pen-shaped body and a vibrating MN head. The head vibrates rapidly via mechanical drive, ensuring even pressure distribution across the skin during application.
MN drug loading for hair regeneration: enhancing therapeutic efficacy
Hair loss can be categorized into the five main types. (1) AGA - the most common form of hair loss, including male and female pattern baldness, associated with genetics and androgen levels; (2) AA - an autoimmune disorder that causes partial or total hair loss. Resting phase hair loss, often grouped with AA in clinical discussion, typically results from stress, malnutrition, postpartum changes, or certain medications, all of which cause large numbers of hairs to enter the telogen (resting) phase and fall out simultaneously; (3) Anagen effluvium (AE) - a less common form of hair loss, usually triggered by certain medications or underlying diseases; (4) Telogen effluvium (TE) - caused by various factors such as acute illness, malnutrition, or medication side effects, leading to an abrupt shift of hair follicles into the telogen phase; (5) Cicatricial alopecia (CA) - permanent hair loss due to the destruction of hair follicles by inflammation or scar tissue. These classifications cover the most common causes and forms of hair loss. MNs have emerged as a promising tool for delivering a range of therapeutic agents to treat different types of alopecia. Table 1 lists the principles of MN-based drug delivery systems used for hair loss treatment.
Microneedles loaded with different compounds that promote hair proliferation
| Principle | Drug molecules or compounds | Microneedle composition | Researcher and reference |
| Inhibit the synthesis of 5α-dihydrotestosterone and reduce androgen | Finasteride | Carboxymethyl cellulose | Kim et al.[44] |
| β-sitosterol | Chitosan | Prabahar et al.[45] | |
| Prolong the growth period and increase hair diameter | MXD | HA | Kim et al.[32] |
| Boost perifollicular vasodilation | MXD and CAP | HA | Chen et al.[48] |
| Degrade androgen receptors in dermal papilla cells | Androgen receptor- PROTAC | HA | Wang et al.[50] |
| Inhibit GSK-3 to stabilizeβ-catenin and activate Wnt/β-catenin signaling | VPA | Carboxymethyl cellulose | Fakhraei Lahiji et al.[62] |
| Micropigmentation of scalp | Black tattoo ink | HA | Lahiji et al.[85] |
| Regulate follicle cycle and promote regeneration | Exocrine + UK5099 (from MSCs) | HA | Yang et al.[67] |
| Accelerate transition from rest to growth | Rapamycin + EGCG nanoparticles | PLGA | Lin et al.[70] |
| Activate Wnt/β-catenin by downregulating SFRP2 | miR-218 | HA | Zhao et al.[71] |
| Remove ROS and promote angiogenesis | CeNZs | HA | Yuan et al.[55] |
| Eliminate ROS | MnPS3-based SOD mimic | HA | Zhang et al.[59] |
| Inhibit DHT synthesis, activate HFSCs, and promote regeneration | Qu, Zn2+, Cu2+ | HA | Zhang et al.[78] |
| Enhance inductive ability of dermal papilla cells | MSCs | PLGA shell + GelMA | Lee et al.[80] |
| Stimulate follicles and induce growth | ADSCs, chitosan, exosomes | PVA tip + HA substrate | Shi et al.[83] |
In most cases, hair loss- particularly AGA - is driven by testosterone, which is converted to dihydrotestosterone (DHT) by the enzyme 5-α reductase[43]. FNS, a 5α-reductase type 2 inhibitor, is approved by the U.S. Food and Drug Administration (FDA) for oral treatment of AGA. However, oral administration can lead to systemic side effects due to its long-term suppression of androgen activity in other tissues, potentially impairing reproductive function and causing male breast development. To address these limitations, Kim et al. developed a MN system that encapsulates FNS powder in dissolvable MNs, enabling direct skin implantation of the drug FNS[44]. Similarly, Prabahar et al. used chitosan-based MNs to deliver β-sitosterol, a plant-derived 5α-reductase inhibitor, to inhibit 5α-DHT synthesis and reduce testosterone-induced alopecia[45]. This approach significantly promoted hair growth in rats and reduced the number of hair follicles in the resting phase. MXD, a potassium channel opener, is FDA-approved for hair loss treatment and works by relaxing vascular smooth muscle, dilating arterioles, and prolonging hair follicle growth while increasing follicle diameter[46]. However, due to poor scalp absorption, MXD requires frequent application, which reduces patient compliance[47]. Studies have shown that HA MNs loaded with MXD significantly enhance local therapeutic efficacy and reduce side effects, achieving results with only 10% of the typical topical dosage[32]. Other approaches include combining nanostructured lipid carriers with MXD and cold atmospheric plasma (CAP) to release nitric oxide, effectively reshaping the hair follicle microenvironment and promoting regrowth[48]. In AGA patients, androgen receptor (AR) levels in dermal papilla cells (DPCs) are elevated, increasing scalp sensitivity to androgens and shortening the hair growth cycle[49]. Wang et al. developed a PROTAC (proteolysis-targeted chimera) that degrades ARs, incorporated into MNs for targeted delivery to DPCs[50]. This approach achieved hair regrowth results comparable to MXD, without systemic toxicity or androgen deficiency-related side effects, making it a promising alternative.
Microneedling has also shown efficacy in treating AA when combined with PRP or triamcinolone. It enhances therapeutic outcomes by releasing growth factors and inducing local immunosuppression. One study showed that PRP stimulates proliferation and differentiation of hair follicle stem cells (HFSCs), and MNs enable efficient local delivery of PRP, reducing pain while preserving efficacy[51]. Another study used a 1.5 mm MN roller followed by topical application of 10 mg/mL triamcinolone, which safely and effectively promoted hair regrowth in AA patients[52].
AE, commonly associated with chemotherapy, may benefit from MN systems delivering antioxidants to mitigate follicular damage. Elevated reactive oxygen species (ROS) in DPCs contribute to premature cellular senescence and inhibit the transition of follicles from the resting to growth phase via androgen and signaling disruptions[53]. Ceria nanocrystals (CeNZs), known for their ROS-scavenging and wound-healing properties[54], were incorporated by Yuan et al. into a DMN system in 2021[55]. This system delivered CeNZs to deep skin layers, improving the oxidative stress microenvironment, reshaping the follicular microvasculature system, and promoting hair regeneration in AGA mice[55]. Superoxide dismutase (SOD), a key enzyme in oxidative stress defense, decomposes oxygen free radicals to protect cells[56]. In 2022,
For TE, MNs can be loaded with drugs that enhance blood circulation and hair follicle nutrition, improve the scalp microenvironment, and facilitate nutrient delivery to hair follicles, thereby inducing the transition from the telogen phase to the anagen phase. Valproic acid (VPA), an anticonvulsant drug, inhibits glycogen synthase kinase (GSK-3), thereby preventing the degradation of β-catenin, stabilizing the Wnt/β-catenin signaling pathway, and promoting the anagen phase of hair follicles[60,61]. In 2018, Fakhraei Lahiji et al. developed a DMN encapsulating VPA (DMN-VPA)[62]. Micro-injury-mediated delivery of DMN-VPA upregulated the Wnt/β-catenin pathway, as well as markers such as alkaline phosphatase, proliferating cell nuclear antigen, claudin protein, and HFSC markers (keratin 15 and CD34), resulting in significantly greater hair density compared to VPA administration alone. Additionally, exosomes, a type of extracellular vesicle (EV), stimulate hair growth by promoting the transition to and prolongation of the anagen phase[63]. Exosomes can activateβ-catenin signaling due to the Wnt proteins present on their surface[64,65]. HFSCs play a key role in regulating the hair growth cycle. UK5099, a pharmacological inhibitor of the mitochondrial pyruvate carrier, activates HFSCs by enhancing glycolysis metabolism[66]. In 2019, Yang et al. employed MN devices to deliver HFSC activators such as exosomes derived from mesenchymal stem cells (MSCs) and UK5099 to modulate hair follicle cycle transitions and stimulate hair regeneration[67], resulting in improved pigmentation and hair regrowth in mice. Rapamycin (RAPA), a macrolide immunosuppressant, promotes the transition from the resting to the growing phase, enhancing hair regeneration[68]. Epigallocatechin gallate (EGCG) supports hair growth by stimulating DPC proliferation and inhibiting apoptosis, thereby prolonging the anagen phase[69]. In 2022, Lin et al. reported a dissolving MN device composed of PLGA that co-delivered nanoparticles of RAPA and EGCG, accelerating the transition to the growth phase and promoting hair regeneration[70]. Secreted frizzled-related protein 2 (SFRP2) inhibits the Wnt/β-catenin signaling pathway. MiR-218 promotes hair follicle transition into the growth phase by downregulating SFRP2 and activating Wnt/β-catenin signaling. Zhao et al. developed HA MNs loaded with miR-218, effectively enhancing hair regeneration by accelerating the transition from telogen to anagen[71].
Trace elements are also crucial for hair follicle development and immune modulation[72]. Zinc ions (Zn2+), essential for maintaining hair shaft diameter[73], are involved in the activity of several Zn2+-dependent enzymes such as alkaline phosphatase and metalloproteinases, which regulate follicular growth. Zinc deficiency can lead to hair thinning[73,74]. Copper ions (Cu2+) stimulate angiogenesis by upregulating vascular endothelial growth factor (VEGF), supporting the vascularization necessary for follicular development[75]. Both Zn2+ and Cu2+ can inhibit 5α-reductase, thereby reducing DHT synthesis[76]. Quercetin (Qu), a flavonoid, can form highly bioactive chelates with various metal ions[77]. In 2022, Zhang et al. developed a nanocomposite MN patch (ZCQ/MN) composed of copper/zinc co-doped mesoporous silica nanoparticles that subcutaneously release Qu, Cu2+, and Zn2+ to promote hair follicle regeneration[78]. Combining organic compounds with bioactive metal ions presents a promising strategy for addressing the multifactorial pathophysiology of AGA.
For CA, MN-assisted delivery of tissue-repairing agents such as stem cells and growth factors shows therapeutic potential. MSCs have been extensively studied in regenerative medicine. EVs secreted by MSCs can induce hair regeneration by enhancing the inductive properties of mouse DPCs[79]. In 2020, Lee et al. proposed a detachable hybrid MN device (d-HMND) for cell delivery, consisting of MNs with an external PLGA shell and an internal GelMA-mesenchymal stem cell mixture (GMM), which significantly promoted hair regeneration in wounded mouse tissue[80]. Adipose-derived stem cells (ADSCs) secrete cytokines that stimulate follicles and promote hair growth[81]. Stem cell-derived exosomes can activate the Wnt/β-catenin pathway, thereby accelerating DPC proliferation, initiating the anagen phase, and increasing hair density[82]. Shi et al. developed a separable MN patch composed of lactate chitosan and ADSC-derived exosomes[83]. This drug-free patch significantly enhanced hair regeneration within seven days and required less frequent application than conventional topical MXD therapy.
For patients unresponsive to pharmacological treatment, MN-assisted scalp micropigmentation (SMP) is an alternative. SMP uses tiny pigment dots injected beneath the epidermis and dermis to simulate the appearance of hair, with effects lasting 1-2 years[84]. In 2019, Lahiji et al. developed a dissolving microneedle (PBM) containing micro-pigment, which enabled precise, minimally invasive pigment injection into the scalp, enhancing scalp coloration and achieving a visually pleasing outcome lasting over one year[85].
Mechanistic insights into MNs promoting hair growth
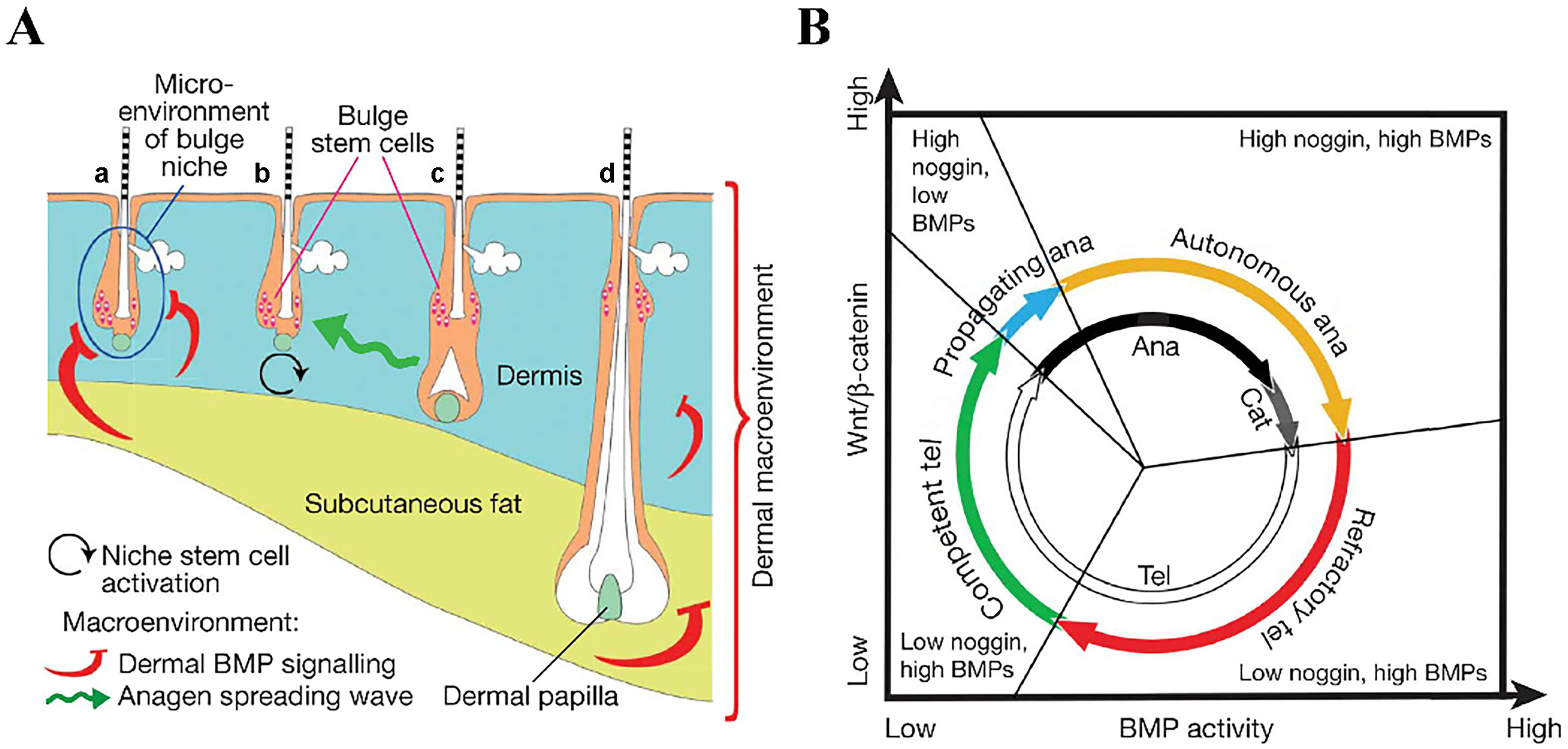
The hair follicle cycle comprises three phases: the growth phase (anagen), the regression phase (catagen), and the resting phase (telogen)[86-88]. The regulation of this cycle is influenced by both the local follicular microenvironment and the broader dermal environment[86,89] [Figure 3]. During the anagen phase, cells in the bulge region proliferate, driving hair follicle development. The catagen phase is characterized by the degeneration of hair follicles, primarily through the apoptosis of follicular keratinocytes. In the telogen phase, the hair follicle root detaches from the dermal papilla and surrounding tissues, anchoring to the hair shaft to form a club hair, which is eventually shed[87]. Studies have shown that the transition from telogen to anagen is associated with the activation of the Wnt/β-catenin and Sonic hedgehog (Shh) signaling pathways, upregulation of lymphoid enhancer-binding factor 1 (LEF-1), and downregulation of bone morphogenetic proteins (BMPs)[86,90].
Figure 3. Schematic diagram of the hair growth cycle phases[86]. (A) Illustration of the bulge niche microenvironment and the interfollicular dermal macroenvironment, including the dermis, subcutaneous fat, and adjacent follicles. Arrows indicate anagen-promoting (black and green) or anagen-inhibiting (red) activities. Hair follicles are shown in different phases: (a) refractory telogen; (b) competent telogen; (c) propagating anagen; and (d) autonomous anagen. The blue circle in (A) marks the intrafollicular microenvironment, color-coded to match (B); (B) New functional phases (outer colored circle) mapped onto classical hair-cycle stages (inner black-and-white circle). Based on their ability to induce growth, anagen is subdivided into propagating (blue, inducing) and autonomous (yellow, non-inducing) phases. Telogen is divided into refractory (red) and competent (green) phases according to follicular responsiveness to regenerative signals. BMPs: Bone morphogenetic proteins.
β-catenin is highly expressed during the anagen phase, initiating follicle formation and hair growth[91].
Kim et al. used MN rollers to repeatedly stimulate the dorsal skin of mice and observed enhanced hair growth. The treatment increased the expression of Wnt3a, Wnt10b, β-catenin, and VEGF proteins, indicating that microneedling may promote hair growth through the activation of Wnt/β-catenin signaling and VEGF-mediated vascular regulation[99]. Nonetheless, microneedling, which involves puncturing the skin down to the dermis, may also cause bleeding, inflammation, and collagen remodeling. Disruption of skin integrity leads to the release of cytokines (IL-1α, IL-8, IL-6, TNF-α, and GM-CSF), accompanied by keratinocyte migration and vasodilation[100].
Currently, few studies have fully elucidated the mechanisms of MN-induced hair regeneration. Most existing research attributes the effect to skin stimulation caused by MN penetration. The following mechanisms are primarily proposed[39,101]: (1) stimulation of collagen production and enhancement of blood circulation; (2) activation of growth factors; (3) facilitation of drug absorption; (4) regulation of the hair growth cycle; (5) improvement of the scalp microenvironment; and (6) activation of HFSCs. As MN applications in hair regeneration continue to advance, additional mechanisms are likely to be discovered.
APPLICATION OF MNS IN PATIENTS WITH SEVERE HAIR LOSS
Use of MNs in hair transplantation surgery: clinical advances
Currently, autologous hair transplantation is widely practiced both locally and globally for hair restoration. This technique, which originated in the 1930s, has undergone nearly 80 years of development and refinement. Two primary methods are used: follicular unit transplantation (FUT) and follicular unit extraction (FUE). FUE is more popular due to its minimally invasive nature and faster recovery time[102]. However, FUE also has shortcomings - it is time-consuming, technically demanding, and sometimes associated with a low graft survival rate. The success of FUE largely depends on the surgeon’s experience and technical proficiency; the longer the grafts remain outside the body, the lower their survival rate, which typically ranges from 54% to 95%[103].
Clinical studies have also explored FUE-based approaches involving reinjection of extracted hair follicle tissues to enhance hair density - referred to as the micro-graft stem cell injection strategy.
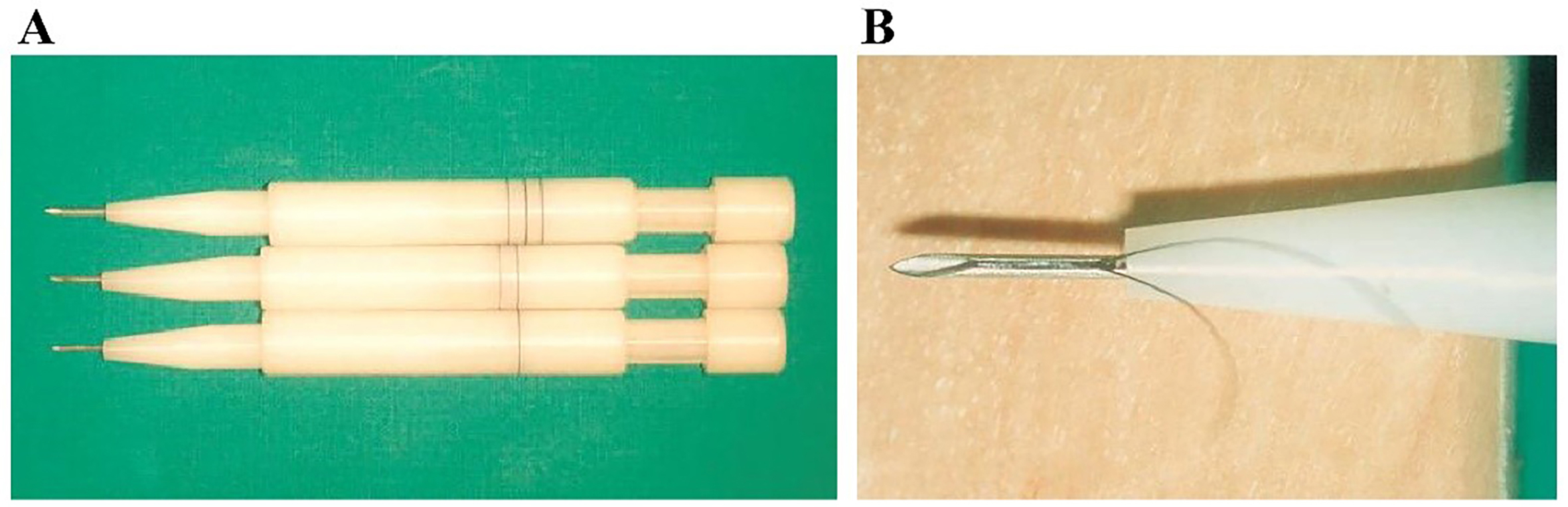
To overcome these limitations, the application of MNs in FUE procedures has emerged as a promising technological advancement. Currently, 18-20G MNs are used to assist with hair implantation, allowing 1-4 follicular units to be implanted per puncture[107]. Implantation tools such as Choi and KNU implanters offer greater efficiency[108]. The KNU implanter [Figure 4], developed in South Korea, significantly enhances the speed and efficiency of hair implantation, achieving survival rates of 92.0% at six months and 90.4% at twelve months post-transplantation[10]. MN-assisted transplantation enables precise follicle implantation into the scalp, minimizing extrusion and mechanical damage seen in traditional techniques, thus improving graft survival. Additionally, this method allows for finer, denser implantation, with more uniform follicle distribution and a more natural aesthetic outcome. In 2011, the first robotic device for skin surgery was approved in the United States, capable of automatically screening and extracting follicular units to alleviate operator fatigue during FUE procedures[109].
Figure 4. Representative image of a microneedle implant pen: (A) KNU implanters of different sizes, developed from the classic Choi implanter; (B) Close-up view of the tip (inset)[10].
Dilemma of tissue-engineered hair follicle regeneration: application prospects
Autologous hair transplantation generally yields good aesthetic results, but the limited availability and applicability of autologous hair restrict its widespread use. Therefore, achieving in vitro hair expansion and orderly regeneration remains an urgent challenge. Recently, HFSCs and organoids have attracted significant interest among researchers. Stem cells from the bulge region of hair follicles can generate interfollicular epidermis, hair follicle structures, and sebaceous glands. Gentile et al. isolated human adult stem cells from hair follicles using in vivo biopsy and mechanical centrifugation and used these cells to treat AGA[110].
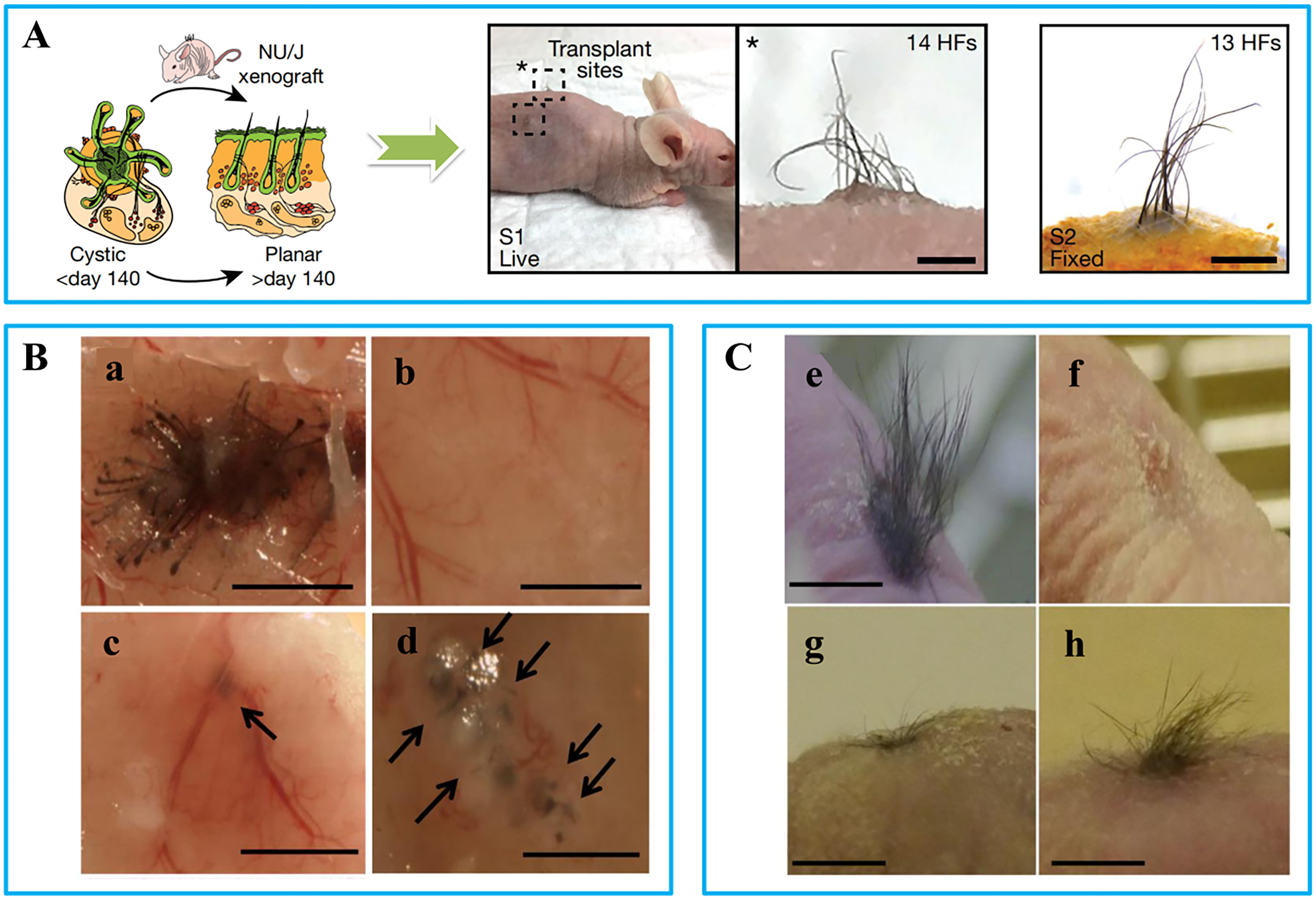
Figure 5. Representative images of different hair transplantation approaches: (A) Skin organ transplantation: images of in vitro cultured skin organoids and hair regeneration before and after transplantation into nude mice[111]; (B) Injection method[112]: (a) Positive controls: freshly isolated dermal and epidermal cells from mouse embryos (E17.5-18.5) were injected; (b) Negative control: only epidermal cells were injected; (c) DPCs treated with FGF2 did not always form hair shafts; (d) DPCs treated with FGF2 + PDGF-AA (10-50 ng/mL) formed hair shafts; (C) Chamber method[112]: (e) Positive control induced hair follicles; (f) Negative control did not induce any follicles; (g) DPCs treated with FGF2 induced sparse hair follicles; (h) DPCs treated with FGF2 + PDGF-AA (20 ng/mL) induced more abundant hair follicles (h). (Scale bar = 200 μm). DPCs: Dermal papilla cells; FGF2: fibroblast growth factor-2; PDGF: platelet-derived growth factor; AA: alopecia areata.
Combining dermal tissue from newborn mouse embryos with epithelial cells can also induce the formation of new hair follicles[11]. This method is more convenient than extracting and expanding HFSCs. In vivo hair follicle regeneration is commonly achieved through injection- or chamber-based methods. Gentile et al. obtained HFSCs via scalp biopsy and mechanical centrifugation, and improved hair density in AGA patients by injecting an autologous hair follicle cell suspension into their scalps[110]. However, this method requires high precision: shallow injections result in poor cell survival, while deeper injections hinder hair growth. Kiso et al. compared the patch assay and the chamber assay for inducing hair regrowth[112]. The patch assay, a type of injection method, involves injecting freshly isolated epidermal and mesenchymal cells from neonatal mice into the hypodermis of recipient mice, resulting in the formation of mature hair follicles. The chamber assay, also known as the mini-chamber method, involves creating wounds on nude mice and filling them with dermal and epidermal cells. In their study, DPCs cultured with PDGF and fibroblast growth factor-2 (FGF2) were used to promote hair regeneration. As shown in Figure 5B and C, although effective in mice, the patch assay leads to disorganized hair growth in the subcutis, while the chamber assay causes additional wounds and lacks control over hair density and direction, limiting its clinical applicability.
To summarize, current tissue-engineered hair regeneration methods, primarily the subcutaneous injection and mini-chamber approaches, face significant limitations. The subcutaneous method shows only modest improvements in hair density, as the lack of proper skin integration causes hair to grow beneath the skin surface without emerging. The mini-chamber method, while promoting substantial hair regrowth, results in clustered, disorganized hair growth and introduces new wounds and scarring. MNs offer a promising solution, as they can puncture the skin effectively to create uniform skin channels. MN arrays, in particular, have the advantage of isometric distribution, making them a useful tool to overcome these challenges. Therefore, developing MN-based techniques that can accurately regulate hair density and growth direction is essential for achieving orderly hair regeneration, bringing tissue-engineered hair follicle regeneration closer to clinical application.
Application of MNs in tissue engineering for hair follicle regeneration: latest progress
3D printing technology offers new options for hair regeneration. Kang et al. developed a 3D-printed scaffold that provides an optimal microenvironment for DPCs to regenerate complete hair follicles, thereby promoting hair growth and enabling control over growth direction[113]. Similarly, Li et al. demonstrated the precise regulation of hair regeneration using a customized microneedle array (MNA) fabricated via 3D printing[114]. However, 3D-printed materials often lack the mechanical properties required to effectively penetrate the skin, requiring wound preparation that may result in scarring. MN materials, by contrast, can be engineered with tunable hardness to form skin-penetrating arrays aligned with hair growth density. Kageyama et al. developed a microporous polydimethylsiloxane (PDMS) mold for the mass production of self-sorted hair follicle germs (ssHFGs) derived from a mixture of mouse epidermal and mesenchymal cell suspensions. These ssHFGs were encapsulated in hydrogel and transplanted into the dorsal skin of nude mice, leading to new hair growth observed after 18 days[115]. Chang et al. further confirmed the viability and proliferative capacity of cells delivered using cryoMNs in mouse models[22]. Collectively, these findings highlight the potential of MNs as tools for cell delivery and the organized regeneration of hair follicles.
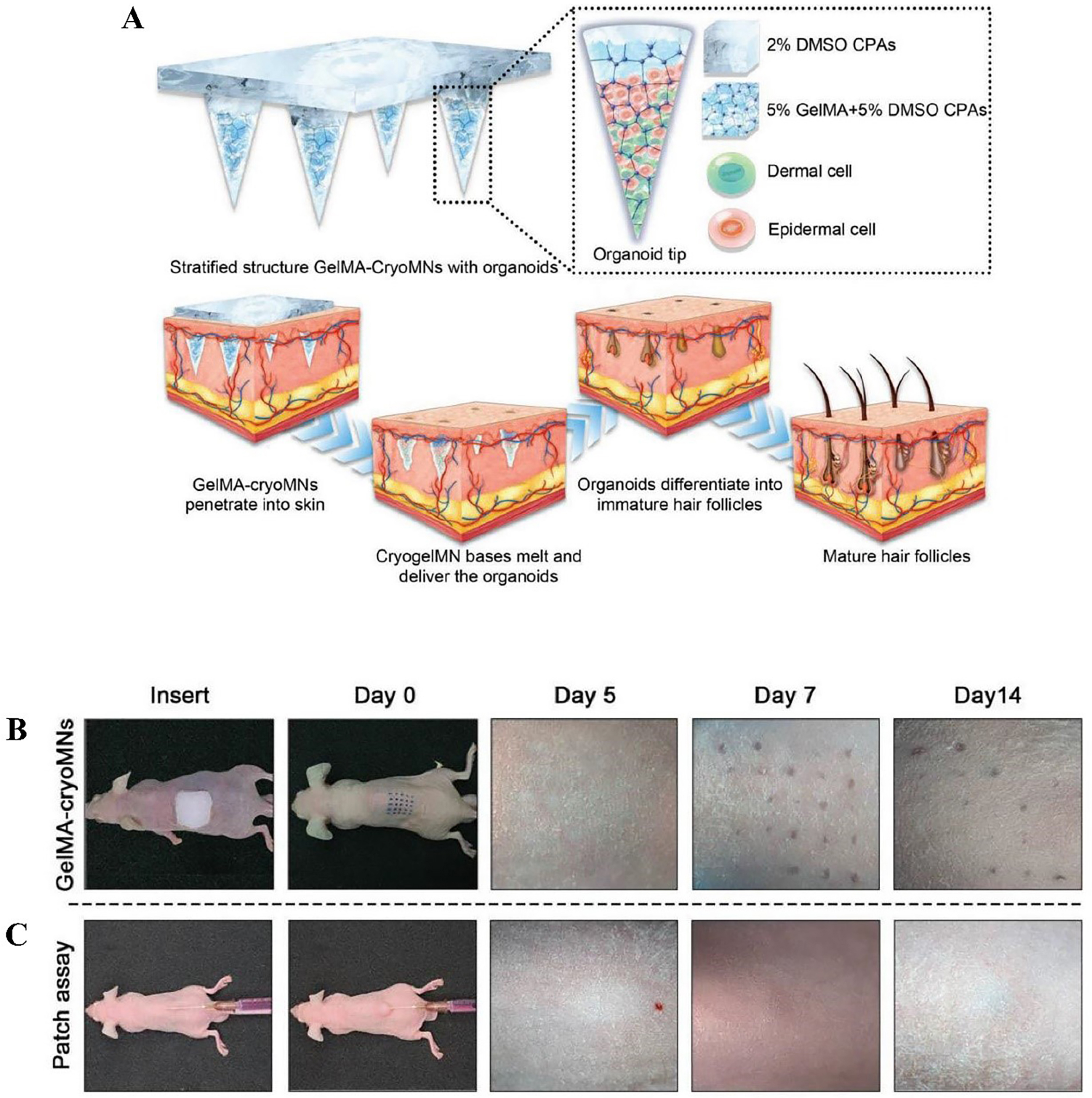
In a recent study, Zheng et al. encapsulated hair follicle seed cells in GelMA hydrogel, followed by gradient freezing to obtain cell-loaded cryoMNs [Figure 6][23]. These were directly injected into the dorsal skin of nude mice, and hair regeneration was observed after 14 days. However, while GelMA hydrogel provides a favorable environment for cell adhesion, it poses challenges for cultivating complex hair follicle organoids. High hydrogel concentrations impede cell aggregation, whereas low concentrations fail to maintain structural integrity, resulting in reduced hair density. These findings underscore the need to optimize hydrogel formulations to support follicle development and improve hair regeneration outcomes.
Figure 6. Organoid-loaded cryoMNs for hair regeneration[23]: (A) Schematic illustration of the transdermal delivery of organoids via GelMA-cryoMNs and subsequent hair follicle differentiation; (B) Organoids loaded into GelMA-cryoMNs; (C) Cells delivered through a patch assay, shown in vertical view on mouse dorsal skin at 0, 5, 7, and 14 days. cryoMNs: Cryo-microneedles; GelMA: gelatin methacryloyl.
SUMMARY AND OUTLOOK
Although MNs are widely used in treating hair loss, their application in hair follicle regeneration remains in the early stages. Drug-loaded MNs can extend the anagen phase of existing hair follicles and promote thicker hair. However, to regenerate new hair follicles from scratch, tissue-engineered approaches are required. MNs can provide a conduit for delivering engineered hair follicles and help regulate the density and orientation of new hair, making them a promising strategy for treating hair loss. Despite their potential, the use of MNs for delivering live cells remains underdeveloped. CryoMNs require cryopreservation, which can compromise cell viability. Moreover, hair follicles injected beneath the skin often fail to emerge through the surface, indicating the necessity of a skin opening during follicle development. The rapid degradation of cryoMNs limits the duration of these openings, which in turn affects regeneration efficiency.
Inspired by core-shell MNs, it may be possible to load viable cells at room temperature while using the shell to maintain a channel for hair growth. However, preserving cell viability prior to transplantation remains a critical challenge. Additionally, MN material selection is key: non-degradable MNs can provide long-lasting channels but raise concerns about foreign body residues and their impact on hair growth. In contrast, biodegradable MNs require precise control over degradation time to balance channel maintenance with safety. Ultimately, if MNs can support hair regeneration with natural density and direction - particularly when combined with hair follicle cell expansion technologies - they may offer a comprehensive solution to hair loss. This could represent the most ideal and effective treatment approach currently under development.
DECLARATIONS
Acknowledgments
All figures in this article are either original creations by the authors or sourced from referenced documents. For externally sourced figures, the necessary copyright permissions have been obtained. The manuscript was authored by the listed contributors, with language refined using AI tools to enhance readability and fluency.
Authors’ contributions
Writing - original draft: Xiao L
writing - review and editing: Xiao L, Zhang F, Chen Y, Tang Y, Usman M, Zhang J
conceptualization: Xiao L, Zhang F
funding acquisition: Zhang J
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (Grant No. 82272285).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Bhatnagar S, Dave K, Venuganti VVK. Microneedles in the clinic. J Control Release. 2017;260:164-82.
2. Ma Y, Dong J, Li M, Du X, Yan Z, Tian W. An antimicrobial microneedle patch promotes functional healing of infected wounds through controlled release of adipose tissue-derived apoptotic vesicles. J Nanobiotechnology. 2024;22:579.
3. Sartawi Z, Blackshields C, Faisal W. Dissolving microneedles: applications and growing therapeutic potential. J Control Release. 2022;348:186-205.
4. Han Y, Qin X, Lin W, et al. Microneedle-based approaches for skin disease treatment. Nanomicro Lett. 2025;17:132.
5. Wu C, Yu Q, Huang C, Li F, Zhang L, Zhu D. Microneedles as transdermal drug delivery system for enhancing skin disease treatment. Acta Pharm Sin B. 2024;14:5161-80.
6. Mubki T, Rudnicka L, Olszewska M, Shapiro J. Evaluation and diagnosis of the hair loss patient: part I. History and clinical examination. J Am Acad Dermatol. 2014;71:415.e1-15.
7. Fertig RM, Gamret AC, Cervantes J, Tosti A. Microneedling for the treatment of hair loss? J Eur Acad Dermatol Venereol. 2018;32:564-9.
8. Gupta AK, Quinlan EM, Venkataraman M, Bamimore MA. Microneedling for hair loss. J Cosmet Dermatol. 2022;21:108-17.
9. Chen Y, Ren T, Wu W, et al. Gas-propelled anti-hair follicle aging microneedle patch for the treatment of androgenetic alopecia. J Control Release. 2025;379:636-51.
10. Lee SJ, Lee HJ, Hwang SJ, et al. Evaluation of survival rate after follicular unit transplantation using the KNU implanter. Dermatol Surg. 2001;27:716-20.
11. Kageyama T, Yan L, Shimizu A, Maruo S, Fukuda J. Preparation of hair beads and hair follicle germs for regenerative medicine. Biomaterials. 2019;212:55-63.
12. He C, Yin M, Zhou H, et al. Magnetic nanoactuator-protein fiber coated hydrogel dressing for well-balanced skin wound healing and tissue regeneration. ACS Nano. 2025;19:1713-31.
13. Chambers R. Microdissection studies, III. Some problems in the maturation and fertilization of the echinoderm egg. Biology Bulletin. 1921;41:318-50.
15. Hashmi S, Ling P, Hashmi G, Reed M, Gaugler R, Trimmer W. Genetic transformation of nematodes using arrays of micromechanical piercing structures. Biotechniques. 1995;19:766-70.
16. Henry S, McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles: a novel approach to transdermal drug delivery. J Pharm Sci. 1998;87:922-5.
17. Matriano JA, Cormier M, Johnson J, et al. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res. 2002;19:63-70.
18. McAllister DV, Wang PM, Davis SP, et al. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci U S A. 2003;100:13755-60.
19. Miyano T, Tobinaga Y, Kanno T, et al. Sugar micro needles as transdermic drug delivery system. Biomed Microdevices. 2005;7:185-8.
20. Kim M, Jung B, Park JH. Hydrogel swelling as a trigger to release biodegradable polymer microneedles in skin. Biomaterials. 2012;33:668-78.
21. Wang J, Ye Y, Yu J, et al. Core-shell microneedle gel for self-regulated insulin delivery. ACS Nano. 2018;12:2466-73.
22. Chang H, Chew SWT, Zheng M, et al. Cryomicroneedles for transdermal cell delivery. Nat Biomed Eng. 2021;5:1008-18.
23. Zheng B, Yang L, Feng S, et al. Organoid-loaded cryomicroneedles for biomimic hair regeneration. Adv Funct Mater. 2024;34:2304950.
24. Li WZ, Huo MR, Zhou JP, et al. Super-short solid silicon microneedles for transdermal drug delivery applications. Int J Pharm. 2010;389:122-9.
25. Li J, Liu B, Zhou Y, et al. Fabrication of a Ti porous microneedle array by metal injection molding for transdermal drug delivery. PLoS One. 2017;12:e0172043.
26. Cormier M, Johnson B, Ameri M, et al. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release. 2004;97:503-11.
27. Boks MA, Unger WW, Engels S, Ambrosini M, Kooyk Yv, Luttge R. Controlled release of a model vaccine by nanoporous ceramic microneedle arrays. Int J Pharm. 2015;491:375-83.
28. Wang PM, Cornwell M, Prausnitz MR. Minimally invasive extraction of dermal interstitial fluid for glucose monitoring using microneedles. Diabetes Technol Ther. 2005;7:131-41.
30. Larrañeta E, Lutton RE, Woolfson AD, Donnelly RF. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater Sci Eng R Rep. 2016;104:1-32.
31. Nguyen TT, Choi JA, Kim JS, et al. Skin immunization with third-generation hepatitis B surface antigen using microneedles. Vaccine. 2019;37:5954-61.
32. Kim MJ, Seong KY, Kim DS, et al. Minoxidil-loaded hyaluronic acid dissolving microneedles to alleviate hair loss in an alopecia animal model. Acta Biomater. 2022;143:189-202.
33. Zeng Y, Wu L, Jiang X, et al. Self-assembled hyaluronic acid nanoparticles delivered by polymeric microneedles for targeted and long-acting therapy of psoriasis. Int J Pharm. 2025;669:125073.
34. Oh JH, Park HH, Do KY, et al. Influence of the delivery systems using a microneedle array on the permeation of a hydrophilic molecule, calcein. Eur J Pharm Biopharm. 2008;69:1040-5.
35. Mohite P, Puri A, Munde S, et al. Hydrogel-forming microneedles in the management of dermal disorders through a non-invasive process: a review. Gels. 2024;10:719.
36. Xu B, Liu H, Yang G, Zhang S, Zhou Z, Gao Y. Novel double-layered PLGA microparticles-dissolving microneedle (MPs-DMN) system for peptide drugs sustained release by transdermal delivery. Int J Pharm. 2025;670:125128.
37. Wang QL, Zhang XP, Chen BZ, Guo XD. Dissolvable layered microneedles with core-shell structures for transdermal drug delivery. Mater Sci Eng C Mater Biol Appl. 2018;83:143-7.
38. You SK, Noh YW, Park HH, et al. Effect of applying modes of the polymer microneedle-roller on the permeation of L-ascorbic acid in rats. J Drug Target. 2010;18:15-20.
39. Dhurat R, Sukesh M, Avhad G, Dandale A, Pal A, Pund P. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
40. Lee YB, Eun YS, Lee JH, et al. Effects of topical application of growth factors followed by microneedle therapy in women with female pattern hair loss: a pilot study. J Dermatol. 2013;40:81-3.
41. Zhang F, Yang YN, Feng JD, et al. Observation on the efficacy of a combined treatment for moderate and severe androgenetic alopecia incorporating electric microneedles. Clin Cosmet Investig Dermatol. 2022;15:2573-81.
42. Yu AJ, Luo YJ, Xu XG, et al. A pilot split-scalp study of combined fractional radiofrequency microneedling and 5% topical minoxidil in treating male pattern hair loss. Clin Exp Dermatol. 2018;43:775-81.
43. Thepphankulngarm N, Manmuan S, Hirun N, Kraisit P. Nanotechnology-driven delivery of caffeine using ultradeformable liposomes-coated hollow mesoporous silica nanoparticles for enhanced follicular delivery and treatment of androgenetic alopecia. Int J Mol Sci. 2024;25:12170.
44. Kim S, Eum J, Yang H, Jung H. Transdermal finasteride delivery via powder-carrying microneedles with a diffusion enhancer to treat androgenetic alopecia. J Control Release. 2019;316:1-11.
45. Prabahar K, Udhumansha U, Elsherbiny N, Qushawy M. Microneedle mediated transdermal delivery of β-sitosterol loaded nanostructured lipid nanoparticles for androgenic alopecia. Drug Deliv. 2022;29:3022-34.
46. Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-94.
47. Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-8.
48. Chen H, Tang X, Huang Y, et al. Remodel the perifollicular microenvironment via Minoxidil-loaded microneedle patch and cold atmospheric plasma for treating androgenetic alopecia. Nano Res. 2024;17:6411-9.
49. Chew EGY, Tan JHJ, Bahta AW, et al. Differential expression between human dermal papilla cells from balding and non-balding scalps reveals new candidate genes for androgenetic alopecia. J Invest Dermatol. 2016;136:1559-67.
50. Wang R, Zhong T, Bian Q, et al. PROTAC degraders of androgen receptor-integrated dissolving microneedles for androgenetic alopecia and recrudescence treatment via single topical administration. Small Methods. 2023;7:e2201293.
51. Ragab SEM, Nassar SO, Morad HA, Hegab DS. Platelet-rich plasma in alopecia areata: intradermal injection versus topical application with transepidermal delivery via either fractional carbon dioxide laser or microneedling. Acta Dermatovenerol Alp Pannonica Adriat. 2020;29:169-73.
52. Chandrashekar B, Yepuri V, Mysore V. Alopecia areata-successful outcome with microneedling and triamcinolone acetonide. J Cutan Aesthet Surg. 2014;7:63-4.
53. Jadkauskaite L, Coulombe PA, Schäfer M, Dinkova-Kostova AT, Paus R, Haslam IS. Oxidative stress management in the hair follicle: Could targeting NRF2 counter age-related hair disorders and beyond? Bioessays. 2017;39:1700029.
54. Wu H, Li F, Wang S, et al. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials. 2018;151:66-77.
55. Yuan A, Xia F, Bian Q, et al. Ceria nanozyme-integrated microneedles reshape the perifollicular microenvironment for androgenetic alopecia treatment. ACS Nano. 2021;15:13759-69.
56. Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. 2021;20:652.
57. Han Y, Tang B, Wang L, et al. Machine-learning-driven synthesis of carbon dots with enhanced quantum yields. ACS Nano. 2020;14:14761-8.
58. Frey NC, Akinwande D, Jariwala D, Shenoy VB. Machine learning-enabled design of point defects in 2D materials for quantum and neuromorphic information processing. ACS Nano. 2020;14:13406-17.
59. Zhang C, Yu Y, Shi S, et al. Machine learning guided discovery of superoxide dismutase nanozymes for androgenetic alopecia. Nano Lett. 2022;22:8592-600.
60. Jo SJ, Choi SJ, Yoon SY, et al. Valproic acid promotes human hair growth in in vitro culture model. J Dermatol Sci. 2013;72:16-24.
61. Lee SH, Yoon J, Shin SH, et al. Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS One. 2012;7:e34152.
62. Fakhraei Lahiji S, Seo SH, Kim S, et al. Transcutaneous implantation of valproic acid-encapsulated dissolving microneedles induces hair regrowth. Biomaterials. 2018;167:69-79.
64. Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036-45.
65. Kost Y, Muskat A, Mhaimeed N, Nazarian RS, Kobets K. Exosome therapy in hair regeneration: a literature review of the evidence, challenges, and future opportunities. J Cosmet Dermatol. 2022;21:3226-31.
66. Flores A, Schell J, Krall AS, et al. Lactate dehydrogenase activity drives hair follicle stem cell activation. Nat Cell Biol. 2017;19:1017-26.
67. Yang G, Chen Q, Wen D, et al. A therapeutic microneedle patch made from hair-derived keratin for promoting hair regrowth. ACS Nano. 2019;13:4354-60.
68. Chai M, Jiang M, Vergnes L, et al. Stimulation of hair growth by small molecules that activate autophagy. Cell Rep. 2019;27:3413-21.e3.
69. Kwon OS, Han JH, Yoo HG, et al. Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG). Phytomedicine. 2007;14:551-5.
70. Lin Y, Shao R, Xiao T, Sun S. Promotion of hair regrowth by transdermal dissolvable microneedles loaded with rapamycin and epigallocatechin gallate nanoparticles. Pharmaceutics. 2022;14:1404.
71. Zhao Y, Tian Y, Ye W, et al. A lipid-polymer hybrid nanoparticle (LPN)-loaded dissolving microneedle patch for promoting hair regrowth by transdermal miR-218 delivery. Biomater Sci. 2022;11:140-52.
72. Almohanna HM, Ahmed AA, Tsatalis JP, Tosti A. The role of vitamins and minerals in hair loss: a review. Dermatol Ther. 2019;9:51-70.
74. Zhang Y, Chang M, Bao F, et al. Multifunctional Zn doped hollow mesoporous silica/polycaprolactone electrospun membranes with enhanced hair follicle regeneration and antibacterial activity for wound healing. Nanoscale. 2019;11:6315-33.
75. Wu C, Zhou Y, Xu M, et al. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials. 2013;34:422-33.
76. Sugimoto Y, López-Solache I, Labrie F, Luu-The V. Cations inhibit specifically type I 5 alpha-reductase found in human skin. J Invest Dermatol. 1995;104:775-8.
77. Kasprzak MM, Erxleben A, Ochocki J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015;5:45853-77.
78. Zhang Z, Li W, Chang D, et al. A combination therapy for androgenic alopecia based on quercetin and zinc/copper dual-doped mesoporous silica nanocomposite microneedle patch. Bioact Mater. 2023;24:81-95.
79. Rajendran RL, Gangadaran P, Bak SS, et al. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci Rep. 2017;7:15560.
80. Lee K, Xue Y, Lee J, et al. A patch of detachable hybrid microneedle depot for localized delivery of mesenchymal stem cells in regeneration therapy. Adv Funct Mater. 2020;30:2000086.
81. Fukuoka H, Narita K, Suga H. Hair regeneration therapy: application of adipose-derived stem cells. Curr Stem Cell Res Ther. 2017;12:531-4.
82. Liu Y, Wang H, Wang J. Exosomes as a novel pathway for regulating development and diseases of the skin. Biomed Rep. 2018;8:207-14.
83. Shi Y, Zhao J, Li H, et al. A drug-free, hair follicle cycling regulatable, separable, antibacterial microneedle patch for hair regeneration therapy. Adv Healthc Mater. 2022;11:e2200908.
84. Rassman WR, Pak JP, Kim J. Scalp micropigmentation: a useful treatment for hair loss. Facial Plast Surg Clin North Am. 2013;21:497-503.
85. Lahiji SF, Um DJ, Kim Y, Jang J, Yang H, Jung H. Scalp micro-pigmentation via transcutaneous implantation of flexible tissue interlocking biodegradable microneedles. Pharmaceutics. 2019;11:549.
86. Plikus MV, Mayer JA, de la Cruz D, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340-4.
88. Chang LY, Plikus MV, Jablonski NG, Lin SJ. Evolution of long scalp hair in humans. Br J Dermatol. 2025;192:574-84.
89. Botchkarev VA, Botchkareva NV, Nakamura M, et al. Noggin is required for induction of the hair follicle growth phase in postnatal skin. FASEB J. 2001;15:2205-14.
90. Chen D, Jarrell A, Guo C, Lang R, Atit R. Dermal β-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development. 2012;139:1522-33.
91. Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533-45.
92. Kulessa H, Turk G, Hogan BL. Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. EMBO J. 2000;19:6664-74.
93. Botchkarev VA, Botchkareva NV, Roth W, et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol. 1999;1:158-64.
94. Wang LC, Liu ZY, Gambardella L, et al. Regular articles: conditional disruption of hedgehog signaling pathway defines its critical role in hair development and regeneration. J Invest Dermatol. 2000;114:901-8.
95. Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635-48.
96. Genander M, Cook PJ, Ramsköld D, et al. BMP signaling and its pSMAD1/5 target genes differentially regulate hair follicle stem cell lineages. Cell Stem Cell. 2014;15:619-33.
97. Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001;107:409-17.
98. Chen Y, Lu Z, Feng J, et al. Novel recombinant R-spondin1 promotes hair regeneration by targeting the Wnt/β-catenin signaling pathway. Acta Biochim Biophys Sin. 2023;55:1213-21.
99. Kim YS, Jeong KH, Kim JE, Woo YJ, Kim BJ, Kang H. Repeated microneedle stimulation induces enhanced hair growth in a murine model. Ann Dermatol. 2016;28:586-92.
100. Ocampo-Garza SS, Fabbrocini G, Ocampo-Candiani J, Cinelli E, Villani A. Micro needling: a novel therapeutic approach for androgenetic alopecia, a review of literature. Dermatol Ther. 2020;33:e14267.
101. Xing Z, Zhang X, Zhao C, et al. Microenvironment-responsive recombinant collagen XVII-based composite microneedles for the treatment of androgenetic alopecia. Acta Biomater. 2025;200:400-15.
102. Jimenez F, Alam M, Vogel JE, Avram M. Hair transplantation: basic overview. J Am Acad Dermatol. 2021;85:803-14.
103. Sharma R, Ranjan A. Follicular unit extraction (FUE) hair transplant: curves ahead. J Maxillofac Oral Surg. 2019;18:509-17.
104. Krefft-Trzciniecka K, Piętowska Z, Pakiet A, Nowicka D, Szepietowski JC. Short-term clinical assessment of treating female androgenetic alopecia with autologous stem cells derived from human hair follicles. Biomedicines. 2024;12:153.
105. Gentile P, Garcovich S, Perego F, et al. Autologous micrografts containing nanovesicles, exosomes, and follicle stem cells in androgenetic alopecia: in vitro and in vivo analysis through a multicentric, observational, evaluator-blinded study. Aesthetic Plast Surg. 2025;49:43-58.
106. Gentile P, Scioli MG, Bielli A, et al. Platelet-rich plasma and micrografts enriched with autologous human follicle mesenchymal stem cells improve hair re-growth in androgenetic alopecia. biomolecular pathway analysis and clinical evaluation. Biomedicines. 2019;7:27.
108. Patwardhan N, Mysore V; IADVL Dermatosurgery Task Force. Hair transplantation: standard guidelines of care. Indian J Dermatol Venereol Leprol. 2008;74:S46-53.
109. Avram MR, Watkins S. Robotic hair transplantation. Facial Plast Surg Clin North Am. 2020;28:189-96.
110. Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4:58.
111. Lee J, Rabbani CC, Gao H, et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature. 2020;582:399-404.
112. Kiso M, Hamazaki TS, Itoh M, Kikuchi S, Nakagawa H, Okochi H. Synergistic effect of PDGF and FGF2 for cell proliferation and hair inductive activity in murine vibrissal dermal papilla in vitro. J Dermatol Sci. 2015;79:110-8.
113. Kang D, Liu Z, Qian C, et al. 3D bioprinting of a gelatin-alginate hydrogel for tissue-engineered hair follicle regeneration. Acta Biomater. 2023;165:19-30.
114. Li R, Yuan X, Zhang L, et al. 3D printing of microneedle arrays for hair regeneration in a controllable region. Mol Biomed. 2023;4:1.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].