Multicomponent porous metal oxide semiconductors for advanced gas sensing
Abstract
Rapid advances in sensing technologies are transforming how humans monitor and interact with their surroundings, driving progress in fields spanning environmental protection, industrial safety, healthcare, food quality control, and intelligent living systems. Within this technological landscape, metal oxide semiconductor (MOS) sensors have emerged as a particularly promising platform, offering high sensitivity, fast response, low cost, and facile integration into diverse device architectures. Recent years have witnessed rapid progress in multicomponent porous MOS sensors, which leverage synergistic effects between different components and hierarchical porous architectures to achieve superior sensing performance. This review provides a comprehensive overview of multicomponent porous MOS gas sensors, encompassing fundamental sensing mechanisms, performance evaluation parameters, and fabrication processes. Particular emphasis is placed on advanced strategies for performance enhancement, including the construction of mesoporous architectures, elemental doping, noble metal functionalization, and heterojunction engineering. Furthermore, recent progress in practical applications is summarized, covering industrial and public safety, environmental monitoring, food freshness evaluation, and disease diagnosis. Finally, future development trends and critical challenges are discussed, highlighting opportunities in high‐throughput synthesis, machine learning‐assisted material design, low‐temperature flexible sensing, multi-parameter intelligent detection, and scalable device integration. This review aims to provide a systematic framework and forward‐looking perspective to inspire the rational design and technological advancement of next‐generation multicomponent MOS gas sensors.
Keywords
INTRODUCTION
In the era of artificial intelligence (AI) and the internet of things, sensor technologies play a pivotal role as essential bridges connecting the material world with human society. Mimicking human sensory organs, modern sensors gather and convey information across multiple spatial scales, significantly extending our capacity to sense and interpret the environment. They have become powerful tools for exploring and transforming nature, driving technological innovation, and supporting societal development.
As a key branch of sensor technology, gas sensors have found widespread applications in diverse fields including disease diagnosis[1,2], environmental monitoring[3], food quality control[4], and industrial safety assurance[5,6]. In recent years, their applications have further expanded into emerging domains including intelligent healthcare and elderly care[7,8], aerospace and military areas[9], and battery safety in electric vehicles[10], as well as various AI-driven monitoring systems[11-13]. Over the past decades, various types of gas sensors have been developed, such as catalytic combustion sensors, electrochemical sensors, thermal conductivity sensors, infrared absorption sensors, paramagnetic sensors, solid electrolyte sensors, and metal oxide semiconductor (MOS) sensors[14]. Among these, MOS sensors have attracted particular attention due to their high sensitivity, fast response, low cost, compact size, and facile fabrication and integration, making them one of the most extensively studied and applied sensor technologies[15-17].
Driven by advances in materials chemistry and nanotechnology, MOS gas sensors have undergone significant evolution, enabling improved sensitivity, selectivity, stability, and response speed. In particular, the integration of multiple components and the construction of porous architectures have emerged as powerful strategies to enhance sensing performance[18-20], meeting the increasing demands of complex application scenarios. The incorporation of dopants, noble metals, or secondary oxides provides a versatile means to tailor electronic structures, defect chemistry, catalytic properties, and surface adsorption behavior[21,22]. Meanwhile, introducing mesoporosity offers high surface areas, abundant accessible active sites, and efficient gas diffusion pathways, which are essential for rapid and sensitive detection[23,24]. Despite rapid progress, the field is now confronted with the challenge of rationally integrating these design strategies to achieve controllable architectures and optimized sensing functions in a systematic manner.
This review provides a comprehensive overview of recent developments in multicomponent porous MOS gas sensors. First, the fundamental aspects of MOS gas sensors, including device fabrication, sensing mechanisms, and performance evaluation metrics, are introduced to provide the necessary background. Next, various performance enhancement strategies are systematically classified and discussed, including mesoporous structure construction (hard-template method, soft-template method, and self-templating method), elemental doping (rare-earth elements, transition-metal elements, and non-metal elements), noble metal modification, and heterojunction engineering (p-n, p-p, and n-n junctions). Special attention is given to how these strategies influence structural features, electronic properties, and surface reactions. Finally, the diverse applications of multicomponent MOS gas sensors in industrial and public safety, environmental monitoring, food quality assessment, and disease diagnosis are summarized. By systematically integrating recent advances in multicomponent design and mesostructure engineering, this review establishes a clear framework for understanding the structure-property-function relationships that govern sensing performance. We also outline current challenges and future research directions, aiming to inspire further innovation towards the rational design of next-generation high-performance MOS gas sensors.
OVERVIEW OF METAL OXIDE SEMICONDUCTOR GAS SENSORS
Manufacture of MOS sensors
The most commonly employed sensor device is the chemiresistive gas sensor manufactured from ceramic tubing. Its structure comprises a porous laminate of sensing material, a heater, and a resistance measurement element (typically a pair of metal electrodes). The manufacturing process comprises several steps. First, a sintered ceramic tube with a gold electrode and platinum wire is prepared. Next, a nickel-chromium alloy coil is inserted into the ceramic tube to serve as a heater for controlling the sensor’s operating temperature. Finally, a uniform coating of sensitive material is deposited onto the surface of the alumina ceramic tube. The manufacture of high-quality gas sensors typically commences with the wet process for preparing fine MOS powder. For instance, in a mesoporous zinc oxide (mZnO)-based sensor, the material is lightly milled to a microcrystalline size of approximately 10 nm[25]. This is then suspended in deionized water within an agate mortar to form a slurry. Subsequently, the slurry is coated onto the ceramic tube surface to form a thick film, with a nickel-chromium alloy coil positioned inside the tube as a heater. To enhance sensor stability prior to testing, the prepared sensor is aged for one week at its optimum operating temperature. The assembled device is illustrated in Figure 1A. The static gas distribution method is employed to test gas response. Within the circuit measuring gas response [Figure 1B], the load resistor (RL) is connected in series with the gas sensor to maintain the voltage across the sensor within its optimal range. The circuit voltage (VC) is set to 5 V, with the output voltage (Vout) representing the load voltage across the load resistor. In a typical sensing process, the test gas is first introduced into the test chamber, where its concentration is adjusted through air dilution. Subsequently, the test gas reacts chemically with oxygen molecules adsorbed on the sensing layer, releasing free electrons. This process causes a decrease in the sensor’s resistance and voltage, leading to an increase in Vout. Figure 1C illustrates the schematic structure of the developed mZnO-based gas sensor.
Figure 1. (A) Assembled ceramic tube device; (B) Gas sensing measurement circuit; (C) Structural schematic of the bypass-heated mZnO-based ceramic tube sensing device. Figure A-C is quoted with permission from Zhou et al.[25]; (D) Schematic diagram of MEMS sensor and its spatial structure; SEM image of the central region of the MEMS chip. Figure 1D is quoted with permission from Yan et al.[26]. mZnO: Mesoporous zinc oxide; MEMS: microelectromechanical system; SEM: scanning electron microscope.
As a miniaturized version of ceramic tube devices, microelectromechanical system (MEMS)-based sensors developed in recent years have garnered significant attention. Various types of MOS sensors fabricated using MEMS technology offer numerous advantages, including compact size, light weight, superior performance, ease of mass production, and low cost. Consequently, numerous studies have commenced employing MEMS technology to manufacture diverse gas sensors. Figure 1D presents a schematic diagram and scanning electron microscope (SEM) image of the MEMS sensor device[26]. Zhu et al. demonstrated a novel self-heating MEMS sensor featuring heterostructured p-CuO/n-SnO2 core-shell nanowires, exhibiting outstanding gas sensing performance towards formaldehyde[27]. The specific fabrication process involves the following steps. First, the prepared p-CuO/n-SnO2 sensing material is uniformly dispersed in deionized water, and then dropped onto the MEMS heating device. After sufficient drying at room temperature, the MEMS device containing the sensitive material was placed in a 45 °C oven for 24 h. Subsequently, the fabricated MEMS device was connected to the external circuit via aluminum wire bonding. Guo et al. prepared three-dimensional (3D) CuO/WO3 hierarchical hollow microspheres assembled from irregular nanosheets (NSs) via ultrasonic wet chemical etching and pyrolysis, and fabricated MEMS sensors based on this material[28]. Compared to the pristine WO3 sensor, the CuO/WO3 sensor exhibited enhanced xylene gas sensing properties, including faster response and recovery, higher sensitivity, good selectivity, and long-term stability. The favorable sensing characteristics of the CuO/WO3 MEMS sensor can be attributed to its unique 3D hierarchical structure and p-n heterojunctions. Wang et al. employed a template-free ultrasonic spray pyrolysis method to synthesize Co-doped In2O3 (Co-In2O3) exhibiting a hollow porous microstructure and abundant active adsorption sites[29]. The resulting Co-In2O3-based toluene sensor, utilizing MEMS technology, enabled operation under conditions of several tens of milliwatts of power.
For MOS as a sensing material, the adoption of MEMS-based microheater devices offers numerous advantages. Firstly, the compact dimensions of such devices enable device miniaturization, thereby enhancing the flexibility and applicability of the sensor. Secondly, the low power consumption of MEMS microheater devices helps extend sensor lifespan and reduce energy expenditure. Thirdly, the easily adjustable temperature of this device enables precise control over the operating temperature of sensors, thereby enhancing their stability and repeatability. Consequently, the MEMS-based microheater device is expected to become the preferred technology for future MOS gas sensors.
Sensing mechanism of MOS sensors
Based on their dominant charge carriers, MOS can be categorized into n-type (where the charge carriers are electrons) and p-type semiconductors (where the charge carriers are holes). For instance, metal oxides including WO3, ZnO, In2O3, SnO2, Fe2O3, TiO2, and MoO3 belong to n-type semiconductors, whereas Co3O4, NiO, CuO, Cu2O, Mn3O4, and Cr2O3 are classified as p-type semiconductors[30]. The sensing mechanisms vary depending on the intrinsic properties of the materials. With the widespread application and in-depth research of gas sensors, discussions on sensing mechanisms have also increased. Scientists have attempted to construct more reasonable and universal theoretical models to explain the MOS sensing mechanism. Researchers have analyzed and interpreted data from multiple perspectives, uncovering numerous distinct mechanisms, including the most prevalent electron depletion layer (EDL) theory[31], hole accumulation layer (HAL) theory[32], bulk resistance control mechanism[33], and gas diffusion control mechanism[34]. These theories encompass certain gas sensing mechanisms within corresponding materials, though significant overlaps exist between them.
Electron depletion layer theory and hole accumulation layer theory
For chemiresistive MOS sensors, the sensing mechanism is primarily attributed to electrical signal changes induced by gas adsorption and desorption. At the microscopic level, when MOS materials are exposed to air, oxygen molecules adsorb onto the surface of the sensing material by capturing electrons from the conduction band, subsequently ionizing into chemically adsorbed oxygen species (such as O2-, O-, or O2- ions). The type of chemically adsorbed oxygen is strongly dependent on the operating temperature (T). At relatively low temperatures (T < 150 °C), O2- is the predominant oxygen species. Between 100 and 300 °C, O- ions are primarily adsorbed onto the MOS surface. At higher temperatures (T > 300 °C), the O2- species become dominant. The formation process of oxygen species can be summarized by[35,36]:
Meanwhile, an EDL with higher resistance than the core region forms on the surface of n-type MOS, or a HAL with lower resistance than the core region forms on the surface of p-type MOS. This results in an increase (or decrease) in the resistance of the n-type (or p-type) MOS. When MOS materials are exposed to an atmosphere containing target gases, redox reactions occur between gas molecules and chemically adsorbed oxygen species. This further results in the release of electrons back into the conduction band of the MOS (for reducing gases) or the capture of additional electrons from the MOS (for oxidizing gases). Accordingly, the thickness of the EDL (or HAL) and the resistance of the MOS change. For instance, when n-type MOS material is exposed to reducing gases (such as acetone), the thickness of the EDL decreases, consequently reducing the resistance of the n-type MOS material [Figure 2A][37]. Conversely, when oxidizing gases are introduced, both the EDL thickness and the resistance of the n-type MOS further increase. For p-type MOS materials, when reducing gases (such as acetone) are introduced, the resistance increases due to the recombination of released electrons and holes, resulting in a decrease in HAL thickness [Figure 2B]. This behavior is the opposite of that observed when oxidizing gases are introduced[38]. Following these processes, the resistance of the MOS material undergoes significant changes, exhibiting a pronounced response to the target gas.
Figure 2. (A) Schematic of the electron depletion layer theory. Mechanistic representation of acetone sensing by an n-type MOS sensor (A1); Initial electronic levels (A2); Changes in the electronic levels of n-type MOS upon exposure to oxygen (A3) and target gases (A4). Figure 2A is quoted with permission from Panigrahi et al.[37]; (B) Core-shell configuration of p-type MOS in vacuum and air (B1); Interaction of p-MOS in the presence of reducing gas CO (B2) and in oxidizing NO2 (B3). Figure 2B is quoted with permission from Kaur et al.[38]; (C) Schematic illustration of gas diffusion processes at different pore sizes; (D) Schematic representation of gas diffusion processes within hollow tin dioxide microspheres at different temperatures. Figure 2C and D is quoted with permission from Wang et al.[34]. MOS: Metal oxide semiconductor.
Bulk resistance control mechanism
The bulk resistance control mechanism fundamentally relies on the resistance variation of the MOS sensors originating from phase transitions within the sensitive materials. The mechanism has a relatively limited applicability, and is only suitable for analyzing gas sensing processes involving γ-Fe2O3 and perovskite-type metal oxides (ABO3), where ABO3 denotes the general chemical formula of perovskite structures.[30]. Wang et al. developed Fe2O3 sensors with distinct α-Fe2O3 and γ-Fe2O3 phase compositions by heating Fe-metal-organic framework (MOF) templates, and analyzed the differing gas sensing mechanisms of the two iron oxides[39]. α-Fe2O3 possesses a relatively stable phase structure, and its sensing mechanism can be explained by a typical oxygen adsorption model. However, the resistance change in γ-Fe2O3 is primarily attributed to alterations in its internal phase structure. γ-Fe2O3 features an inverse spinel structure, wherein the octahedral voids (Fe2+ and half Fe3+ ions) typically feature a vacant cation site (□) to compensate for the increased positive charge. When exposed to a reducing gas environment, the Fe3+ ions within the octahedral sites are reduced to Fe2+, causing the material to transform into Fe3O4. Owing to the existence of the (Fe2+-Fe3+-Fe2+-Fe3+) structure within Fe3O4, electron migration becomes significantly facilitated, resulting in a material whose resistivity is ten orders of magnitude smaller than that of γ-Fe2O3. Upon re-exposure to air, Fe3O4 re-oxidizes to γ-Fe2O3, with its electrical resistance increasing accordingly. It should be noted that the redox reactions occurring during gas sensing are reversible, involving only simple phase transitions without any crystal structure transformation. These mechanisms are expressed as[40,41]:
where Th denotes the tetrahedral interstitial, □ represents a cation vacancy, Oh signifies the octahedral interstitial, and x is the reduction degree of Fe3+.
Gas diffusion control mechanism
In gas sensing processes, two key components are typically involved: the sensitive material and the target gas. The aforementioned EDL, HAL and bulk resistance control mechanisms primarily focus on the physical and chemical properties of the sensing materials, whereas the gas diffusion control mechanism places greater emphasis on the transport behaviors of the gas within sensing materials. The most significant factor influencing gas diffusion is the material morphology. As early as the 1990s, scientists proposed the theory that gas diffusion governs the sensitivity of MOS sensors[42-44]. Indeed, this theory serves as a complement to the former two mechanisms, offering a more comprehensive explanation for the variations in physical parameters during gas sensing. By studying the diffusion process of gases through material surfaces, we can gain deeper insights into the operating principles of gas sensors and provide theoretical guidance for designing and optimizing novel intelligent sensors.
Wang et al. constructed pore channel models through research on SnO2 microspheres, comprehensively explaining the relationship between surface chemical reactions (SCR) and gas diffusion, along with their impact on gas sensing performance. Based on Knudsen diffusion theory and reaction kinetics, the gas diffusion rate (Dk) and SCR rate (k) are respectively expressed by[34]
where r denotes the pore radius, R is the gas constant (8.314 J·mol-1·K-1), T represents the temperature, M is the molecular weight, A is the pre-exponential factor, and Ea denotes the activation energy. Based on the two equations, k is primarily determined by T. At low temperatures (T < 180 °C), the SCR rate is relatively low, making it the rate-determining step controlling the overall reaction. Furthermore, the entire process occurs on the surface, with the external surface area the primary factor controlling gas sensing performance. At elevated temperatures (T > 260 °C), the increase in Dk is less pronounced than that of k, rendering the process diffusion-controlled, in which pore size exerts a more significant influence on gas sensing performance. At intermediate temperatures (180 °C ≤ T ≤ 260 °C), gas diffusion and SCR compete in equilibrium, with the entire system operating under dual control. Overall, as temperature increases, diffusion control progressively supplants SCR control. Based on this, this work proposes a pore channel model to analyze the diffusion of gas molecules [Figure 2C]. At low temperatures (T < 180 °C), the mean free path of gas molecules exceeds the pore diameter, thereby impeding their diffusion into the channels and causing most responses to occur outside the channels. Although both diffusion and SCR rates are relatively low, the SCR rate plays a decisive role. As temperature increases, the mean free path of gas molecules gradually diminishes to match the pore size, enabling more molecules to diffuse into the pore channels and react with oxygen species, forming a transition state. At high temperatures (T > 260 °C), the mean free path becomes shorter than the pore diameter, enabling greater molecular diffusion. However, the increase in k value far exceeds the Dk value, ultimately leading to the emergence of a diffusion-controlled state. To further elucidate gas sensing behaviors, the authors developed a hollow sphere (HS) model to describe the interplay between SCR and gas diffusion [Figure 2D]. At low temperatures (T < 150 °C), the oxygen species adsorbed on the SnO2 surface are predominantly less reactive O2-, rendering SCR inactive and thus limiting the gas sensing reactions. A portion of the target gas undergoes partial oxidation on the outer surface, while the residual gas diffuses into the pore interior to perform chemical reactions, including partial oxidation, complete oxidation, and ionization of minor oxidation products. In this case, gas diffusion progressively becomes the rate-determining step. Although the gas diffusion control mechanism requires further quantitative analysis and optimization of multiple factors, it has been extensively applied to evaluate the effect of morphology on the sensing performance of MOS materials. A detailed discussion on the construction and regulation of mesoporous architectures, including pore size, morphology and hierarchical design, will be presented in Section Constructing mesoporous metal oxides.
Assessment parameters of MOS sensors
The performance of MOS sensors can be evaluated using a variety of parameters, including but not limited to sensitivity, selectivity, response/recovery times, stability, reversibility, optimal operating temperature, detection limit, and manufacturing cost.
Sensitivity
Sensitivity (S) is one of the key indices for evaluating sensor performance, reflecting the response capability of the sensing material towards the target gas. For chemiresistive gas sensors, the output is the response value (R), defined as the ratio of the sensor’s resistance under the target gas atmosphere to its resistance in air. Taking reducing gases as an example, for n-type semiconductors, R = Ra/Rg; whereas for p-type semiconductors, R = Rg/Ra, where Ra denotes the sensor’s resistance in air, and Rg represents the sensor’s resistance in the target gas. The situation is reversed for oxidizing gases. The distinction between sensitivity and response is not always clear-cut, as both describe the extent of resistance variation. Sensitivity (S) can be expressed in alternative ways:
ΔR denotes the difference between Ra and Rg.
Selectivity
Selectivity refers to a gas sensor’s ability to accurately identify specific gases in the presence of mixed gases. Traditionally, selectivity is defined as the ratio of the sensor’s response towards the target gas to its response towards interfering gases[45]. This implies that the sensor should exhibit a distinctive response solely to the target gas, without interference from other gases. In practical applications, gas sensors often operate within complex working environments, where numerous interfering gas molecules are present during detection. Achieving selective detection of specific gases in mixed atmospheres is paramount, directly influencing the reliability and accuracy of sensors in real-world scenarios. Enhancing selectivity thus represents a significant research focus in gas sensor design and deployment.
Response/recovery time
Response and recovery times are crucial metrics for evaluating sensor performance, indicating how quickly the sensor’s signal rises upon exposure to the target gas and returns to baseline once the gas is removed. Response time (tres) and recovery time (trev) are defined as the times required for a gas sensor to achieve a 90% change in resistance following gas introduction or removal, respectively[46]. In practical applications, shorter response and recovery times indicate that sensors can respond rapidly to gas changes and return quickly to their original state, thereby ensuring the promptness and accuracy of monitoring. Therefore, optimizing response and recovery kinetics is crucial for achieving efficient gas detection, particularly in applications requiring timely responses, such as industrial safety monitoring. Generally speaking, sensing materials with higher porosity possess more gas diffusion pathways, allowing gas molecules to penetrate the material interior more readily, thereby facilitating the rapid response and recovery[47,48].
Stability
Stability refers to the ability of a gas sensor to maintain a consistent and stable signal output after prolonged operation. This is influenced by multiple factors, including material composition, ambient humidity, and operating temperature. The stability of a sensor is one of its most crucial characteristics in practical applications, as it directly influences the sensor’s reliability and lifetime. During prolonged operation, poor sensor stability may result in fluctuating or erroneous output signals, thereby diminishing the reliability and usability. Consequently, when designing and selecting sensors, it is essential to thoroughly consider and enhance their stability to ensure long-term, consistent operation under varying environmental conditions and to deliver accurate and dependable gas detection results.
Reversibility
Reversibility denotes the ability of a gas sensor to restore its baseline state once the target gas concentration returns to normal levels. This property is essential for ensuring reliable and stable sensor operation over time. Inadequate reversibility can cause cumulative measurement errors and undermine detection accuracy, thereby affecting both operational continuity and precision. Therefore, reversibility serves as a key criterion for evaluating sensor performance and practical applicability.
Optimum operating temperature
The optimum operating temperature is defined as the temperature at which a sensor achieves its maximum response to a given gas concentration. At this point, both sensitivity and response dynamics are optimized, enabling accurate and rapid target gas detection. In practice, operating temperatures should be kept as low as possible, since elevated temperatures increase fabrication and energy costs and may induce material degradation or unstable sensing behaviors. Therefore, determining the optimum operating temperature requires a careful balance between performance, energy efficiency, material stability, and practical application demands.
Detection limit
The limit of detection (LOD) is the lowest concentration of a target gas that can be reliably distinguished from the blank under defined conditions, commonly using an S/N (Singal/Noise) ≈ 3 or 3σ criterion. In practice, the LOD is obtained from the calibration curve as LOD = 3σ/m, where m is the slope and σ is the standard deviation of the blank (or replicate low-level measurements). A lower LOD indicates superior detectability at trace levels and is critical for high-sensitivity applications. For example, food spoilage releases marker gases such as hydrogen sulfide (H2S) at parts-per-billion (ppb) concentrations[49], whereas breath biomarkers for early disease screening (e.g., acetone) often occur at parts-per-million (ppm) to ppb levels[50]. Accordingly, achieving low detection limits has substantial practical significance.
Impact factors on MOS sensors’ performance
The performance of gas sensors is primarily determined by several key factors, including the composition, microstructure and grain size of the sensing material.
Composition
Different MOS materials exhibit distinct intrinsic characteristics, including surface properties, carrier concentration, defect types, terminal groups, bandgap, and electronic structure. These factors influence gas-solid interactions in different ways, thereby governing gas sensing performance[51,52]. Rational design and selection of appropriate material systems are therefore essential for achieving high sensitivity and selectivity. In gas sensing, n-type MOS (e.g., WO3, In2O3, SnO2, ZnO) are generally preferred over p-type counterparts[53]. Typically, n-type MOS show more pronounced resistance variations upon gas adsorption, which is attributed to their higher density of grain boundaries[54]. In contrast, p-type MOS usually display weaker resistance changes because parallel conduction pathways at interparticle interfaces diminish the overall modulation effect. Moreover, the sensitivity of p-type MOS sensors has been shown to scale only with the square root of that of n-type MOS sensors[55]. Despite this limitation, many p-type MOS materials, such as CuO, NiO, and Co3O4, remain widely used owing to their excellent catalytic activity[54-56-60]. For materials with wide band gaps, inter-band electron hopping becomes difficult, resulting in smaller resistance changes upon gas adsorption. Conversely, materials with narrow band gaps possess intrinsically high carrier concentrations, which reduce the relative modulation induced by adsorption, often leading to negligible signal changes. Finally, regardless of whether n-type or p-type MOS are employed, single-component materials often suffer from limited sensitivity and severe cross-sensitivity. The synergistic combination of multiple metal oxide components may also induce interfacial interactions, whose detailed roles, including heterojunction formation and interfacial charge modulation, will be systematically discussed in Section Heterojunction engineering.
Microstructure
The electrical and chemical properties of sensing materials can be tuned by tailoring their microstructural morphology, such as into nanowires, nanotubes, nanoparticles (NPs), or porous frameworks[61]. Modifying these structures enables faster and more concentrated interactions between the sensing material and target gases, thereby markedly enhancing sensor sensitivity. In general, materials with larger surface areas exhibit higher response levels due to the increased number of active sites available for gas-solid interactions. Moreover, porous structures with interconnected channels facilitate gas diffusion within the sensing layer, improving contact between the target gas and the material, thereby enhancing reaction efficiency[62,63]. Consequently, nanostructured and porous materials are widely employed to increase surface area and improve sensor performance.
Grain size
Grain size is a critical factor influencing variation in resistance and overall gas sensing performance. Numerous studies have demonstrated its impact on the sensing behavior of metal oxides[64-67]. As shown in Figure 3, sensing materials consist of partially sintered grains connected by necks, which further assemble into larger aggregates through grain boundaries. At the grain surface, adsorbed oxygen molecules withdraw electrons from the conduction band and trap them in ionic form, inducing band bending and generating electron-depleted space-charge layers. When the grain size becomes comparable to or smaller than twice the thickness of the space-charge layer, sensor sensitivity increases markedly[68]. Xu et al. explained this behavior using a semi-quantitative model[69]. Depending on the relationship between grain size (D) and the width (L) of the space-charge layer induced by chemisorbed ions, three regimes can be distinguished. (i) D >> (indicates far exceeding) 2L. Conductivity is dominated by internal charge carriers and follows an exponential dependence on barrier height. In this case, the effect of charges generated by surface reactions is relatively minor; (ii) D ≥ 2L. Space-charge layers at grain necks form constricted conduction channels within aggregates. Conductivity is determined by both interparticle barriers and the channel cross-sectional area, making it highly sensitive to variations in surface charge; (iii) D < 2L. The space-charge layer extends throughout the entire grain, depleting most mobile carriers. Conductivity is then governed primarily by intergranular transport, and even small amounts of surface charge can induce large changes in overall conductivity. Therefore, when the grain size approaches the critical dimension, the material exhibits exceptionally high sensitivity to ambient gas molecules.
Figure 3. Schematic illustration of the effect of grain size on MOS sensor sensitivity. (A) D >> 2L; (B) D ≥ 2L; (C) D < 2L. This figure is quoted with permission from Sun et al.[68]. MOS: Metal oxide semiconductor.
STRATEGIES TO ENHANCE SENSING PERFORMANCE
In the field of gas sensing, MOS materials have been widely employed owing to their excellent sensing properties. With ongoing technological progress and increasing environmental demands, the performance requirements for gas sensors have become more stringent. Modern gas sensors are expected to deliver high sensitivity, excellent selectivity, fast response and recovery, long-term stability, and low power consumption. However, single-component MOS materials used in resistive sensors often fail to meet all these criteria simultaneously. To overcome this limitation, extensive efforts have been devoted to improving their sensing performance, leading to the development of several effective strategies. These include the construction of mesoporous metal oxides[70,71], elemental doping[72,73], noble metal loading[21,74], and heterojunction engineering[75-77], which will be discussed in the following sections.
Constructing mesoporous metal oxides
Among various nanomaterials, mesoporous metal oxides constitute a distinctive class of functional porous materials with tunable pore sizes ranging from 2 to 50 nm. Owing to their adjustable pore structures and compositions, large specific surface areas and pore volumes, unique electronic structures, and facile surface modification, they have attracted significant attention in gas sensing[15,78]. The high surface area and highly crystalline frameworks offer abundant active sites and reaction interfaces, promoting gas adsorption and surface reactions, and thereby enhancing sensor sensitivity. Meanwhile, well-interconnected mesoporous channels improve gas diffusion and mass transfer, increasing the likelihood of gas-solid interfacial interactions. This accelerates adsorption and catalytic reactions, further boosting sensitivity and lowering detection limits. In addition, their highly crystalline frameworks facilitate charge-carrier transport and the detection of carrier-density changes, leading to faster response and recovery. These characteristics make mesoporous metal oxides promising candidates for advanced semiconductor gas sensors with high sensitivity, low detection limit, and rapid response-recovery rate. Consequently, the construction of mesoporous metal oxides is an effective strategy for enhancing gas sensing performance.
Various synthetic strategies, including hard-template (nano-casting), soft-template, and self-templating methods, have been developed to control the morphology and dimensions of mesoporous metal oxides. By selecting appropriate preparation techniques, their mesostructures can be precisely tailored, thereby optimizing gas sensing performance.
Hard-template method
The hard-template method, also known as nano-casting, typically employs pre-synthesized mesoporous materials such as mesoporous silica or carbon as sacrificial scaffolds. The inorganic precursor is impregnated into the template channels to fill the mesopores, followed by thermal treatment to convert it into the corresponding crystalline metal oxide. Subsequent removal of the template yields the desired mesoporous metal oxide framework [Figure 4A][70]. This method offers several advantages. First, it avoids the use of structure-directing agents and bypasses the complex hydrolysis-condensation of inorganic precursors, making it highly versatile for synthesizing various mesoporous materials. Second, mesoporous silica and carbon templates are chemically and thermally stable, and their well-defined channels can be readily infiltrated by appropriate precursors. As a result, the hard-template strategy has been successfully applied to fabricate diverse mesoporous materials, including metal oxides[79], metal sulfides[80], metal nitrides[81], and metal carbides[82]. For example, ordered mesoporous WO3 (mWO3) structures have been obtained using KIT-6 as a template [Figure 4B][79]. Moreover, Deng et al.[70] synthesized a series of ordered mesoporous transition metal oxides, such as Co3O4, NiO, Fe2O3, Cr2O3, and Mn3O4, by employing ordered mesoporous silicas [ Mobil Composition Of Matter N. 41 (MCM-41), Korea Advanced Institute of Science and Technology-6 (KIT-6), and Santa Barbara Amorphous-15 (SBA-15)] as hard templates.
Figure 4. (A) Schematic illustration for the casting process of a key with a mold (A1) and nanocasting using ordered mesoporous materials as the hard template (A2). Figure 4A is quoted with permission from Deng et al.[70]; (B) TEM images of mesoporous WO3 synthesized using KIT-6 as the template (B1, B2); HRTEM image of the ring-like structure, with the inset showing the corresponding FFT pattern (B3); uncoupled framework model (B4). Figure 4B is quoted with permission from Rossinyol et al.[79]; (C) Schematic illustration of the EIAA strategy. Figure 4C is quoted with permission from Jianget al.[24]; (D) Schematic illustration of the one-pot direct synthesis of PB-b-PDMAEMA/H3[PMo12O40] nanocomposite films. Figure 4D is quoted with permission from Lunkenbein et al.[85]; (E) Schematic illustration of the citrate ligand-assisted EISA method for synthesizing crystalline mZnO. Figure 4E is quoted with permission from Zhou et al.[25]; (F) Schematic illustration of the synthesis routes for mesoporous metal oxides via metal carbonate intermediates. Figure 4F is quoted with permission from Eckhardt et al.[86]; (G) TEM images of multicomponent mesoporous metal oxides prepared with acetate chelation assistance. Figure 4G is quoted with permission from Fan et al.[87]. THF: Tetrahydrofuran; TEM: transmission electron microscopy; HRTEM: high-resolution transmission electron microscopy; FFT: fast Fourier transform; EIAA: evaporation-induced aggregate assembly; PDMAEMA: poly(2-(dimethylamino)ethyl methacrylate); H3[PMo12O40]: phosphomolybdic acid; mZnO: mesoporous zinc oxide.
However, the rigid structural constraints of templates such as mesoporous silica and carbon make it difficult to flexibly tune the pore characteristics of the resulting materials, including their dimensions, types, window sizes, and connectivity. In particular, template removal typically requires hazardous chemicals such as hydrofluoric acid or hot potassium or sodium hydroxide solutions, which pose environmental risks and health hazards to operators. Therefore, the hard-template method is associated with high costs, complex procedures, and poor environmental compatibility, limiting its suitability for large-scale production.
Soft-template method
The soft-template method is considered one of the most effective and versatile strategies for fabricating ordered mesoporous materials through controlled co-assembly. Classical soft-templating is primarily realized via evaporation-induced self-assembly (EISA) or evaporation-induced aggregate assembly (EIAA) strategies [Figure 4C][24]. In these processes, weak interactions between inorganic precursors and organic surfactants (e.g., block copolymers) are crucial. These interactions cooperatively drive complex hydrolysis-condensation through multiple forces, including hydrogen bonding, electrostatic interactions, and covalent bonding, leading to the formation of uniform organic-inorganic micelles. As solvents such as tetrahydrofuran, acetone, or methanol evaporate, the mixed micelles pack densely to generate ordered mesoporous frameworks. Despite its advantages, synthesizing ordered mesoporous metal oxides remains challenging due to the intrinsically weak inorganic-surfactant interactions, the limited control over hydrolysis-condensation, and the structural instability of porous networks during high-temperature treatment[83]. To overcome these limitations, numerous strategies have been developed to strengthen inorganic-organic interactions and regulate precursor hydrolysis-condensation behaviors.
Specifically, the interactions between inorganic species and surfactants can be strengthened by selecting appropriate precursors and organic polymer templates. For example, Zhang et al. employed a laboratory-synthesized amphiphilic block copolymer, polystyrene-b-poly(4-vinylpyridine) (PS-b-P4VP), as the structure-directing agent and tetrabutyl titanate (TBOT) as the titanium source to prepare nitrogen (N)-doped ordered mesoporous TiO2[84]. Strong chelation between the pyridine nitrogen and titanium enabled the formation of an ordered mesoporous TiO2 framework with high crystallinity, without the need for additional additives during synthesis. Similarly, Lunkenbein et al. used the diblock copolymer poly(butadiene-block-2-(dimethylamino)ethyl methacrylate) (PB-b-PDMAEMA) and phosphomolybdic acid (H3[PMo12O40]) to fabricate mesoporous films, leveraging strong electrostatic interactions between protonated poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA) segments and PMo12O403- anions [Figure 4D][85].
Small-molecule ligands can act as “bridges” that strengthen coordination between inorganic species and surfactants, thereby promoting organic-inorganic co-assembly. For example, Zhou et al. used citric acid to assist the formation of mesoporous ZnO by enhancing the interaction between Zn2+ ions and poly(ethylene oxide) (PEO) segments [Figure 4E][25]. Citric acid induces partial hydrolysis of zinc nitrate to form zinc citrate, which then binds to ethylene oxide segments through hydrogen bonding. Similarly, Eckhardt et al. employed citric acid as a chelating agent to form stable metal-citrate complexes, which subsequently interact with block copolymers to generate ordered mesophases, yielding ZnO and Co3O4 films with ordered mesopores [Figure 4F][86]. Fan et al. further showed that controlled coordination of metal ions with acetic acid produces nanoscale particles with uniform condensation kinetics, where acetate ligands bridge amphiphilic copolymers and inorganic species[87]. Replacing hydrolysable alkoxy groups with acetate lowers the cross-linking density within the gel network, enabling the synthesis of multicomponent mesoporous oxides [Figure 4G]. Overall, tailored ligands serve as effective molecular linkers, guiding the formation of well-organized mesostructures.
The rapid hydrolysis-condensation of inorganic precursors, such as metal chlorides and alkoxides, often induces phase separation and agglomeration, resulting in disordered structures. To overcome these issues, various modifiers, including hydrochloric acid, nitric acid, and acetylacetone, have been introduced to regulate hydrolysis kinetics. For example, Li et al. used hydrochloric acid as a modifier and poly(ethylene oxide)-b-polystyrene (PEO-b-PS) as a template to fabricate ordered mWO3 with a highly crystalline framework[47]. HCl suppresses the hydrolysis of WCl6 while protonating PEO segments, thereby strengthening hydrogen bonding between hydrophilic tungsten species and the template. Similarly, Wei et al. synthesized mesoporous Al2O3 using aluminum acetylacetonate as the precursor and nitric acid as a modifier[88]. Nitric acid slows hydrolysis, whereas acetylacetonate ligands form chelated complexes that further stabilize Al species. Chelating agents also inhibit aggregation during hydrolysis. Xiao et al. employed acetylacetone to retard SnCl4 hydrolysis, forming acetylacetonate-stabilized tin hydroxide nanoclusters (NCs) with controllable sizes suitable for subsequent co-assembly with block copolymers[89]. An alternative strategy involves the direct co-assembly of preformed nanocrystals with amphiphilic block copolymers, thereby bypassing complications associated with precursor hydrolysis[78]. Corma et al. demonstrated this approach by co-assembling monodisperse CeO2 NPs with PEO20-PPO70-PEO20 (P123) to produce mesostructures exhibiting excellent ordered hexagonal symmetry and exceptional thermal stability up to 700 °C[90]. This strategy has been extended to other oxides such as In2O3[91], TiO2[91], ZnO[92], CuO[92], Mn3O4[93], ZrO2[94], and hybrid oxides including ITO[91] and MnFe2O4[93].
Commercial Pluronic surfactants composed of non-ionic PEO and poly(propylene oxide) (PPO), such as P123[90] and PEO102-PPO65-PEO102 (F127)[95], have been widely used in the synthesis of mesoporous materials. However, their low thermal stability makes it difficult to obtain highly crystalline frameworks. High-temperature annealing often leads to partially crystalline porous structures or even the collapse of metal oxide frameworks. To address this limitation, amphiphilic block copolymers with higher thermal stability and molecular weight, such as PEO-b-PS[47], PS-b-P4VP[84], polystyrene-block-poly(2-vinylpyridine) (PS-b-P2VP)[96], and poly(isoprene)-block-poly(ethylene oxide) (PI-b-PEO)[97], have been employed to prepare mesoporous metal oxides with large pore diameters and high crystallinity. Lee et al. first reported a combined soft-hard template co-assembly (CASH) method using PI-b-PEO as the structure-directing agent[97]. In this process, samples were initially calcined under an inert atmosphere. The PEO segments decomposed readily upon heating, whereas the thermally stable polyisoprene (PI) segments [each containing two s orbital and two p orbitals hybridized (sp2)-hybridized carbons] carbonized to form rigid amorphous carbon, which acted as a scaffold to support the intermediate oxide walls. Subsequent calcination in air removed the residual carbon, yielding highly crystalline mesoporous metal oxides. More recently, our group has further advanced the CASH method by employing PEO-b-PS as templates. A series of mesoporous metal oxides, including WO3[47], TiO2[98], SnO2[89], ZnO[92], CuO[92], and Al2O3[88], have been successfully synthesized through gas-liquid interfacial assembly.
Furthermore, incorporating soluble phenolic formaldehyde resin (resol) and silicate species into the framework has been shown to effectively prevent structural collapse during high-temperature processing. Inspired by this concept, Li et al. developed a pore-engineering strategy using hydrophilic resol as a sacrificial carbon source to synthesize ordered mWO3 with well-connected bimodal pores and crystalline pore walls[99]. In this system, resol preferentially interacts with PEO segments and acts as a binder that links tungsten species with the PEO-b-PS template. During thermal treatment, both the carbonized resol and the copolymer form a rigid matrix that stabilizes the pore structure, yielding highly crystalline hierarchical mWO3. Zhou et al. reported the controlled synthesis of silica-filled mesoporous ZnO[100]. Citric acid was employed as a chelating agent to strengthen the weak interactions between the zinc precursor and the PEO-b-PS template. Simultaneously, tetraethyl orthosilicate (TEOS)-derived silicate oligomers interacted with citric acid through hydrogen bonding, enabling co-assembly with the zinc precursor and providing additional structural support during high-temperature processing.
These sophisticated assembly strategies, including ligand-assisted soft-templating, substitution of inorganic salts with preformed nanocrystals or NCs, sp2 carbon support, and amorphous component (phenolic resin or silicate)-assisted assembly, enable more controllable construction of mesoporous MOS materials with tunable pore architectures and precisely engineered surface properties, paving the way for high-performance gas sensor fabrication.
Self-template method
MOFs represent a distinctive class of porous organic-inorganic hybrid materials constructed through the coordination assembly of metal ions or clusters with organic ligands. Owing to their tunable porosity, high surface areas, compositional diversity, and relatively straightforward synthesis, MOFs have attracted considerable attention in recent years. Representative families include the zeolitic imidazolate frameworks (ZIFs)[101], the Materials of the Lavoisier Institute (MIL) series[102], and the University of Oslo (UIO) series[103]. These materials have been widely applied in gas separation, sensing, catalysis, and energy storage and conversion[104]. Upon high-temperature calcination in air, the metal centers within MOFs are converted into crystalline metal oxides. At the same time, the organic ligands decompose into gaseous products such as CO2 and NO2, thereby forming mesoporous structures. Since this self-templating pyrolysis strategy requires no additional templating agents, it has emerged as an attractive alternative route for synthesizing mesoporous MOS materials.
Mesoporous MOS derived from MOFs largely retain the structural features of their parent frameworks, and a variety of mesoporous MOS sensing materials with tunable morphologies have been obtained by precisely regulating MOF precursors. Wang et al. synthesized copper-based MOF [Hong Kong University of Science and Technology-1 (HKUST-1)] crystals with distinct polyhedral shapes by adjusting the amount of lauric acid as a growth modulator, and subsequent thermal treatment produced Cu2O/CuO sensing materials with octahedral, truncated octahedral, and cubic morphologies[105]. Among these, the octahedral structure with the largest specific surface area exhibited the best ethanol sensing performance. Jang et al. constructed a chrysanthemum-like layered ZnO/Co3O4 composite by in situ coupling rod-like ZIF-67 with sheet-like ZIF-8, followed by calcination[106]. This hierarchical structure, featuring abundant p-n heterointerfaces and high porosity, significantly enhanced acetone sensing, achieving an Ra/Rg of 29.0 to 5 ppm acetone, far exceeding that of pure Co3O4 rods (Ra/Rg = 1.05 at 5 ppm acetone). Li et al. further utilized MOFs as self-templates to synthesize uniform hollow La2O3/In2O3 nanotubes, which exhibited excellent triethylamine (TEA) sensing performance with a high Ra/Rg value of 458.13 to 50 ppm at 120 °C[107]. Such excellent performance can be attributed to the synergistic modulation of the electronic structure and the abundance of acidic sites. Using bimetallic MOFs as precursors, Qin et al.[108] prepared a series of ternary Co-M-O NSs (M = Cu, Mn, Ni, Zn) with ultrahigh surface areas and homogeneous compositions [Figure 5A], which displayed excellent CO sensing performance, including fast response-recovery rates, high stability, and good selectivity. Koo et al. developed PdO-ZnO/ZnCo2O4 HSs by depositing CoZn bimetallic ZIF onto polystyrene spheres, encapsulating Pd NPs within the cavities, and subsequently performing calcination[109]. The resulting p-ZnCo2O4/n-ZnO heterostructure facilitated electron sensitization, thereby endowing the HSs with outstanding acetone-sensing performance. When ternary phases fail to form or stoichiometry is suboptimal, bimetallic oxide composites can still be obtained, as exemplified by In2O3/ZnO porous hollow nanocages derived from InZn-MOF[110], NiO/CuO hydrangea-like composites from NiCu-BTC[111], and NiO/NiCo2O4 multilayer HSs from NiCo-BTC[112]. Moreover, noble metal NPs can be uniformly incorporated into oxide matrices by calcining catalyst-encapsulated MOFs (M@MOFs). For example, Koo et al. used ZIF-8 to support Pd NPs (Pd@ZIF-8), which were subsequently embedded into electrospun fibers and thermally treated to yield Pd@ZnO-WO3 nanofibers (NFs) with excellent toluene sensing properties[113]. In a follow-up study, the same group synthesized PdO-Co3O4 hollow nanocages by calcining Pd@ZIF-67[114], which showed high acetone responses due to the uniform dispersion of PdO catalysts and improved gas accessibility within the hollow structures.
Figure 5. (A) Schematic illustration of ternary metal oxide nanosheet synthesis (A1), and TEM images of Co-Cu-O (A2), Co-Mn-O (A3), Co-Ni-O (A4), and Co-Zn-O (A5). Figure B is quoted with permission from Qin et al.[108]; (B) Schematic illustration of the synthesis of ZnO and Al2O3 (B1); SEM image (B2) and TEM image (B3) for Zn-tannic acid coordination polymer; SEM image (B4) and TEM image (B5) for Al-tannic acid coordination polymer. Figure 5B is quoted with permission from Wang et al.[116]. ZIF: Zeolitic imidazolate framework; MOF: metal-organic framework; NS: nanosheet; TEM: transmission electron microscopy; SEM: scanning electron microscope.
In addition to MOFs, plant-derived polyphenols, as a naturally abundant, nontoxic, and environmentally friendly biomass, have recently emerged as powerful precursors for constructing functional mesoporous nanomaterials[115]. Owing to their strong chelating ability toward metal ions, plant polyphenols readily coordinate with various metal species through metal-catechol complexation, forming stable metal-phenolic coordination polymer networks. Upon subsequent calcination, the polyphenol scaffold decomposes into CO2 and H2O, while the coordinated metal centers undergo oxidation and crystallization, producing mesoporous metal oxides. Because the limited amount of metal species cannot fully inherit the morphology of the parent metal-phenolic framework, extensive mesopores and internal cavities are generated during pyrolysis.
Wang et al. developed a general metal-polyphenol self-templating strategy for synthesizing mesoporous metal oxide microspheres with diverse compositions (Al2O3, ZnO, Fe2O3, Co3O4, and CuO) and highly crystalline frameworks [Figure 5B][116]. Owing to the universal chelation capability of polyphenols toward a wide range of metal ions, this method enables the synthesis of binary, ternary, and even high-entropy mesoporous metal oxides. Using tannic acid as a universal ligand, Wang et al. further demonstrated the fabrication of binary (Fe-Co, Ni-Co, Ni-Zn oxides), ternary (Ni-Co-Zn and Ni-Co-Mn oxides), and quinary (Ni-Co-Fe-Cu-Zn oxides) mesoporous nanospheres[117]. Depending on the intrinsic crystallization temperature and thermal evolution of different metal species, various architectures, such as solid[118], hollow[117], and multi-shell[119] mesoporous structures, can be obtained. These features arise from the nonuniform shrinkage and structural reorganization of metal-phenolic precursors during calcination. Moreover, plant polyphenols exhibit rich structural diversity and tunable chemical functionality. By employing different polyphenols as organic ligands, the morphology of metal-phenolic coordination networks can be flexibly tailored into nanospheres, NFs, NSs, and polyhedra, which are subsequently inherited by the derived mesoporous metal oxides.
Mesoporous semiconductor oxides derived from metal-phenolic self-templating possess high surface area, abundant porosity, compositional tunability, and diverse morphologies, making them highly promising for gas sensing applications[120]. For instance, Feng et al. used polyphenols as molecular glues to co-assemble glutathione-protected Au NCs with Sn species, forming hybrid metal-phenolic nanospheres[121]. After calcination, the Au NCs were unexpectedly converted into atomically dispersed Au single atoms embedded in the mesoporous SnO2 framework. The resulting sensor exhibited an exceptionally high response (587.3) to 2 ppm 3-hydroxy-2-butanone (3H-2B) at 50 °C, an enhancement of 183.5-fold compared with pristine mesoporous SnO2. Additionally, the optimal operating temperature was effectively reduced from 100 °C to 50 °C. Similarly, Li et al. employed the same self-templating process to synthesize Au/Pd dual-atom-sensitized mesoporous SnO2 for H2 detection, achieving an ultralow detection limit (70 ppb), fast response (100 s), and strong resistance to CO, NO, H2S, and SO2 interference[122]. Importantly, polyphenols serve as versatile molecular glues that interact with diverse substrates through hydrophobic interactions, hydrogen bonding, electrostatic forces, π-π stacking, and covalent coupling[123]. After thermal treatment, mesoporous metal oxide films can be deposited directly onto different substrates, offering opportunities for in situ solution-grown, structurally stable multicomponent MOS devices. Furthermore, as naturally abundant biomass molecules, polyphenols can be produced on a large scale, providing a promising foundation for scalable manufacturing of mesoporous metal oxide materials.
Thus, the self-templating strategy has opened new avenues for the rational design of mesoporous MOS materials. By tailoring MOF and metal-phenolic precursor structures and carefully controlling synthesis parameters, the size, morphology, composition, and electrical conductivity of mesoporous MOS can be precisely tuned. Materials synthesized through this approach exhibit unique physicochemical properties and hold great promise for high-performance gas sensing applications.
Overall, the construction of mesoporous architectures remains a fundamental and highly effective strategy for enhancing the gas sensing performance of MOS. Hard-template, soft-template, and self-templating approaches each offer distinctive advantages [Table 1]. The hard-template method enables precise structural replication and well-defined pore ordering, but typically involves multistep procedures and environmentally hazardous template-removal processes. The soft-template approach provides greater versatility and tunability, allowing fine control over pore size and channel configuration. However, it requires meticulous regulation of intermolecular interactions and often faces challenges in maintaining structural integrity during high-temperature calcination. Self-templating routes, including MOF- and metal-polyphenol-derived strategies, feature simplified synthesis and rich compositional adaptability, although the final morphology inherently depends on the structure and chemistry of the precursor framework. In practical applications, the choice among these methods must balance the target pore architecture, desired crystallinity, processing complexity, and cost considerations.
Comparison of mesoporous construction methods
| Strategy | Template type | Pore ordering | Framework crystallinity | Cost & scalability | Advantages | Limitations | Performance enhancement effect |
| Soft-template | Surfactants, block co-polymers | High ordering | Medium | Low cost, scalable | Flexible, universal, tunable | Low thermal stability | Constructing large specific surface areas and interpenetrating pore channels, increasing gas adsorption sites and catalytic active sites |
| Hard-template | Ordered mesoporous silica/carbon | High ordering | High | Higher cost, multi-step procedure | Precise pore control | Harsh template removal procedure | Providing high specific surface areas and uniform diffusion channels, facilitating enhanced sensitivity and response speed |
| Self-template | Metal-organic frameworks | Low ordering | High | Depending on the precursor type | Available for diverse components and morphologies | Strong template dependency | Inheriting the porosity of precursor templates, providing large specific surface areas and abundant active sites, with synergistic component effects |
Elemental doping
Elemental doping, which introduces foreign elements into host materials, has been shown to markedly enhance the sensing performance of MOS. Doping not only tailors the electronic structure but also affects structural characteristics such as grain size and morphology, thereby modulating the overall sensing behavior. Rare-earth elements, transition metals, and non-metal elements can all act as effective dopants. Through judicious selection and design, dopants enable precise control over the electronic and structural properties of MOS, allowing their sensing performance to be optimized for diverse application scenarios.
Rare-earth element doping
Rare-earth elements (REs, including La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, and Yb) possess a unique 4f-electron configuration. Owing to their exceptional electrical conductivity, luminescence, and catalytic properties, they have been widely employed as effective dopants in catalysis and sensing applications[124-126]. Rare-earth oxides typically exhibit high oxygen-ion mobility, favorable catalytic activity, and strong surface alkalinity, which enhance gas sensing performance, particularly in terms of response and selectivity. In addition, rare-earth doping can reduce grain size and increase oxygen vacancy (Ov) concentrations, thereby further improving the sensing characteristics of MOS. Extensive studies have demonstrated that rare-earth elements play a pivotal role in optimizing the gas sensing properties of MOS.
Liu et al. synthesized Ce-doped ordered mWO3 via a straightforward in situ co-assembly approach. Ce doping effect can modulate the coordination environment of W atoms, significantly enhancing Ov and forming Wδ+-Ov sites[127]. Therefore, the resulting Ce-doped WO3 exhibits outstanding H2S sensing performance at low operating temperatures (150 °C), with a high response value of 381 (for 50 ppm), rapid response dynamics (6 s), excellent selectivity, moisture resistance, and good long-term stability [Figure 6A]. Zhao et al. employed an electrospinning strategy to synthesize a series of one-dimensional In2O3 NFs doped with various rare-earth elements (RE-In2O3, RE = Y, La, Nd, Ho, and Tm)[128]. All RE-In2O3 materials exhibited enhanced formaldehyde responses compared to pristine In2O3. Among these, Y-In2O3 demonstrated the highest responsivity and excellent selectivity, which was attributed to the elevated Fermi level and increased surface alkalinity of In2O3, resulting in enhanced oxygen chemisorption and formaldehyde adsorption. Bai et al. prepared pristine In2O3 and RE (Ce, Tm, Eu, Er, and Tb)-doped In2O3 nanotubes via electrospinning. Rare-earth doping markedly improved ethanol sensing, although the extent of enhancement varied among different dopants. Mechanistic analyses indicated that doping modifies the types and distribution of adsorbed oxygen species on the material surface, thereby boosting gas sensing activity[129]. Wang et al. synthesized pristine SnO2 and Tb-doped SnO2 nanotubes with different doping concentrations using uniaxial electrospinning[130]. Tb doping induced pronounced morphological changes, including increased surface roughness, reduced nanotube diameter, and smaller SnO2 grain size. Notably, the 7 mol% Tb-SnO2 nanotubes exhibited the highest response to ethanol, which was attributed to the synergistic effects of reduced grain size, defect generation, structural disorder, enhanced surface alkalinity, and increased roughness introduced by doping. Li et al. fabricated pure MoO3 and Ce-doped MoO3 nanoribbons via a one-step hydrothermal method[131]. Ce doping not only increased the response toward trimethylamine (TMA) but also reduced the optimal operating temperature. The authors proposed that substitution of Mo sites by Ce atoms promotes Ov formation, which facilitates oxygen adsorption and improves sensing performance. Furthermore, Lee et al. have reported several humidity-insensitive sensing materials, including CeO2-loaded In2O3 HSs[132], Tb-doped SnO2 yolk-shell microspheres[133], and Pr-doped In2O3 macroporous microspheres[134]. They attributed this humidity resistance behavior to the +3/+4 redox transitions of multivalent rare-earth elements (Ce, Pr, Tb), which promote the removal of surface hydroxyl groups, the regenerative adsorption of oxygen, and electron consumption [Figure 6B and C]. These processes enhance sensor stability and gas sensing performance by establishing a water-poisoning-resistant reaction pathway.
Figure 6. (A) Sensing responses of the Ce-X/mWO3 sensor to 50 ppm H2S at various temperatures (A1); Sensing mechanism of Ce-X/mWO3-based gas sensors for H2S detection (A2). Energy band structure and electron-transfer process of Ce-X/mWO3 sensitive material exposure in air and H2S-air mixture (A3). Figure 6A is quoted with permission from Liu et al.[127];( B) Gas responses of pure In2O3 (B1) and 11.7 Ce-In2O3 (B2) hollow spheres exposed to 20 ppm acetone (Ace), CO (C), ammonia (A), H2 (H), and toluene (T) at 450 °C in dry and humidity (r.h. 80%); Polar plots of gas responses of pure In2O3 (B3)and 11.7 Ce-In2O3 (B4) hollow spheres exposed to various reducing gases in dry (red) and r.h. 80% (blue). C: Self-refreshing of the In2O3 sensing surface by the CeO2 nanoclusters. Water vapor inflow (C1), chemisorption (C2), desorption (C3), and oxygen ion regeneration (C4). Figure 6B and C is quoted with permission from Yoon et al.[132]. ppm: Parts-per-million.
Transition metal element doping
Transition metals constitute another important class of dopants for enhancing the gas sensing performance of MOS. Owing to their comparable atomic radii to those of common MOS hosts (e.g., W, In, Sn, Zn), many transition metals can be readily incorporated into the lattice through substitutional doping. Such doping not only modulates lattice parameters and oxygen adsorption but also alters defect and carrier concentrations, thereby regulating the overall sensing behavior. Previous studies have demonstrated the effectiveness of various transition metal-based dopants, including V[135], Cr[136,137], Mn[138], Fe[139], Co[140,141], Ni[142,143], Cu[144], and Mo[145,146].
Chen et al. synthesized Co-doped SnO2 sensing materials with a 3D anti-opalescent structure[141]. Among these, the Co-SnO2 sensor with a Co/Sn atomic ratio of 1:24 exhibited the best performance, showing ultrahigh sensitivity, fast response, and excellent selectivity toward low concentrations of formaldehyde (200 °C) and acetone (225 °C), respectively. Mechanistic investigations revealed that cobalt doping narrows the bandgap, elevates the Fermi level, and introduces vacancy defects through the reactions
where,
The electronic sensitization effect induced by transition metal doping can also occur in p-type MOS. Li et al. synthesized pure NiO and Cr-doped NiO NPs via a hydrothermal method and systematically investigated their gas sensing properties[137]. The 25 at% Cr-NiO sensor exhibited high sensitivity (Rg/Ra = 10.6 vs. 100 ppb), excellent selectivity, and good stability for benzyl mercaptan detection. As a p-type semiconductor, NiO conducts through holes. Substitutional Cr3+ doping at Ni2+ sites follows a charge compensation mechanism, as given below:
where
Simultaneously, oxygen molecules oxidize Ni2+ to Ni3+, generating abundant reactive O- species that amplify carrier concentration variations (Δp). Moreover, ionic species such as Ni2+ act as oxygen adsorption sites, forming reactive oxygen species that catalyze surface redox reactions, thereby further increasing Δp. From the perspective of electronic sensitization, the t2g orbitals of Ni overlap with p orbitals of oxygen, causing Pauli repulsion and facilitating partial electron transfer between metal centers and lattice oxygen. Due to the Oh symmetry of the octahedral MO6 unit, Ni2+ and Cr3+ have valence configurations of (t2g)6(eg)2 and (t2g)3(eg)0, respectively. Cr3+, with unfilled t2g orbitals, acts as an electron acceptor, whereas Ni2+, with a fully occupied (t2g)6 configuration, induces e--e- repulsion with the oxygen p orbitals, leading to electron transfer from Ni2+ to Cr3+. This process promotes the formation of Ni3+ species and enhances their electron affinity. The authors proposed that surface high-valence Ni plays a pivotal role in gas redox reactions, while doped Cr regulates carrier concentration and Cr-O-Ni charge transfer, synergistically improving gas sensing performance. Similar phenomena have been reported for Fe doping. Wang et al. synthesized a unique nest-like Fe-NiO structure, whose sensor exhibited a rapid response rate (26 s), short recovery time (5 s), and excellent selectivity toward TEA[139]. At 260 °C, its response to 50 ppm TEA reached 63, a 21-fold enhancement compared with pristine NiO. The improved performance was attributed to the distinctive nest-like architecture and the incorporation of Fe ions into the NiO nanocrystals.
Non-metal doping
The incorporation of non-metallic impurity atoms (e.g., C, N, Si, and S) into MOS frameworks has also been demonstrated to significantly enhance gas sensing performance. Compared with metallic dopants, non-metallic elements can induce the formation of amorphous species, improve thermal stability, enhance selectivity and sensitivity, and lower operating temperatures. Therefore, the rational design of non-metal doping strategies is of great importance for optimizing the performance of MOS sensing materials.
Carbon, an essential non-metal widely used in catalysis and energy conversion, can act as an effective dopant to modulate the micro/nanostructure and physicochemical properties of sensing materials, including localized electron distribution, surface active sites, and electrical conductivity. Raghu et al. synthesized C-doped TiO2 with abundant (101) crystal facets using urea as the carbon source via a mild pyrolysis process [Figure 7A][147]. The resulting material exhibited a markedly improved ethanol sensing response (34.8%) compared with pristine TiO2 (8.5%). Both substitutional and interstitial carbon doping generated abundant active sites, Ov, and chemisorbed oxygen species, which accelerated interfacial catalytic reactions. Moreover, graphitic carbon improved electrical conductivity and reduced the bandgap, thereby lowering the ethanol sensing operating temperature from 250 °C to 150 °C. Similarly, Zhao et al. developed a facile template-free solvothermal route to synthesize C-doped TiO2 NPs (~ 30 nm) without an additional carbon source[148]. The C-doped TiO2 exhibited outstanding alcohol sensing performance, with sensitivity increasing alongside carbon chain length. In particular, its response to n-pentanol was ~ 5.4 times higher than that of undoped TiO2. This improvement was attributed to enhanced conductivity, accelerated electron transfer, and increased surface-adsorbed oxygen, which collectively lowered the operating temperature and boosted sensitivity.
Figure 7. (A) Time-dependent ethanol gas sensing performances of TiO2 and C40-TiO2 sensor performances at 150 °C (A1); Ethanol gas sensing mechanism on pristine TiO2 and C40-TiO2. Figure 7A is quoted with permission from Raghu et al.[147]; (B) molecular structure of N-doped In2O3 (B1); SEM (B2) and TEM (B3) image of N-doped In2O3; The response transients of sensors to 10 ppm NO2 (B4); The linear fitting to the relationship between response and concentrations for In2O3 and N-doped In2O3 (B5). Figure 7B is quoted with permission from Mo et al.[150]; (C) Acetone sensing performance and mechanism of 3D cross-stacked Si-doped WO3 nanowire arrays. Figure 7C is quoted with permission from Ren et al.[153]; (D) Schematic illustration of the synthesis of the Ti3C2Tx MXene, sulfur-doped Ti3C2Tx MXene, and preparation of the MXene sensors (D1); Dynamic response curve of sulfur-doped Ti3C2Tx sensors for toluene at room temperature (D2). Figure 7D is quoted with permission from Shuvo et al.[156]. VOC: Volatile organic compound; TEM: transmission electron microscopy; SEM: scanning electron microscope; ppm: parts-per-million.
Notably, carbon doping can also modify crystalline phases in WO3, thereby improving gas sensing performance. Wang et al. synthesized three-dimensionally ordered macroporous/mesoporous C-doped WO3 using polystyrene spheres as templates followed by post-heating[149]. Carbon atoms diffused into interstitial sites of the WO3 lattice, inducing the formation of ε-phase WO3. Benefiting from this unique crystal structure, the C-doped WO3 sensor exhibited high responsivity and selectivity toward acetone.
Beyond carbon doping, nitrogen incorporation into porous metal oxides can also markedly improve their gas sensing performance through distinct mechanisms. Mo et al. reported a novel N-doped indium oxide gas sensor, which shows a remarkable response to 10 ppm NO2 at room temperature, with outstanding long-term stability (over 30 days) and rapid response/recovery times (5/16 s) [Figure 7B][150]. Abbasi et al. employed density functional theory (DFT) calculations to investigate the adsorption behavior of NO2 molecules on pristine and N-doped anatase TiO2 NPs[151]. Their results showed that NO2 adsorption on N-doped TiO2 is energetically more favorable than on pristine TiO2. Specifically, the nitrogen atom in NO2 preferentially binds to Ov and doped nitrogen sites on the TiO2 surface, while the oxygen atoms exhibit higher affinity for five-coordinated Ti atoms. Moreover, NO2 molecules are more easily oxidized to NO3- due to Ti-O bond cleavage between the TiO2 lattice and NO2. These factors collectively result in superior sensing performance of N-doped TiO2 compared with undoped NPs. Zhang et al. further reported a simple EIAA method for the in situ synthesis of N-doped ordered mesoporous TiO2[84]. The incorporation of nitrogen atoms into the TiO2 lattice led to smaller grain sizes and increased Ov concentrations, both of which enhance gas sensing activity. The resulting ordered mesoporous N-TiO2 sensor exhibited a response of 17.6 toward 250 ppm acetone, an 11.4-fold improvement compared with undoped TiO2. These improvements were mainly attributed to the open pore structure, large specific surface area, and abundant Ov. Additionally, nitrogen doping introduces impurity energy levels that lower the Fermi level of TiO2[152], thereby facilitating electron transfer from adsorbed target molecules to N-TiO2.
Silicon (Si)-doped metal oxides exhibit markedly enhanced gas sensing performance. Notably, ε-WO3, a metastable crystalline phase with exceptional acetone selectivity, can be stabilized by incorporating Si atoms into the framework. A representative example is the versatile directional orthogonal assembly strategy reported by our group[153]. In this method, PEO-b-PS is co-assembled with various Keggin-type heteropolyacids, followed by calcination-induced structural transformation. This approach enables the fabrication of heteroatom-doped MOS 3D multilayer cross-nanowire architectures featuring well-interconnected frameworks and uniform nanowire spacing. Si-doped WO3 nanowires synthesized through this method exhibit the ε-WO3 phase owing to the in situ introduction of Si from silicotungstic acid. Gas sensors based on these nanowires demonstrate excellent acetone sensing characteristics, including high sensitivity (LOD = 10.0 ppb), outstanding selectivity, fast response and recovery, and good stability. This superior performance is attributed to the combined effects of large surface area, abundant catalytic active sites, efficient electron transport along the nanowires, and the high electric dipole moment of ε-WO3
Sulfur (S) doping represents another effective strategy for enhancing the gas sensing performance of semiconductors, primarily by promoting charge transfer between oxides and sulfides. Shuvo et al. reported a S-doped Ti3C2Tx MXene gas sensor, which shows a greater toluene gas sensing performance compared to their undoped counterparts with unique selectivity and long-term stability [Figure 7D][156]. S doping leads to a 214% and 312% increase in sensing response for 1 ppm and 50 ppm toluene, respectively, compared to undoped materials. Simultaneously, it exhibits a pronounced response to extremely low concentrations of 50 ppb toluene, demonstrating its exceptionally low detection limit. Li et al. synthesized biomorphic mesoporous SnO2 using biomass carbon as a template and subsequently introduced sulfur through chemical vapor deposition to obtain S-doped SnO2[157]. The S-terminated sites of the resulting SnS2 phases act as active reaction centers for NO2 adsorption, thereby improving sensing performance. The S-doped SnO2 sensor exhibited an excellent response of 57.38 toward 100 ppm NO2, with a fast response time of 1.60 s and a detection limit of 10 ppb at room temperature. This performance enhancement was attributed to the formation of S-Sn-O bonds, which serve as electron-transfer bridges, reduce interfacial state density, increase carrier concentration, and generate more chemisorbed oxygen, thus accelerating NO2 reaction kinetics at room temperature. Xu et al. further achieved S-doped surface modification of SnO2 by sintering flower-like SnS2[158]. The obtained flower-like mesoporous S-doped SnO2 exhibited ultrahigh sensitivity to NO2 at low operating temperatures (50 °C), with an Rg/Ra value of 600 toward 5 ppm and a detection limit of 50 ppb (Rg/Ra = 11). The enhanced performance was attributed to the modified surface chemistry induced by S doping, which endowed the SnO2 surface with higher catalytic reactivity. In situ Raman spectroscopy and DFT calculations confirmed that S-doped SnO2 possesses superior catalytic activity and strong adsorption capability, and that its sensing performance is closely related to the S doping level.
Elemental doping represents a powerful strategy for tailoring the intrinsic physicochemical properties of MOS materials to achieve high-performance gas sensing. Different dopants operate through distinct mechanisms [Table 2]. Rare-earth elements, characterized by their partially filled 4f orbitals and variable oxidation states, are particularly effective in modulating band structures, enhancing surface alkalinity, and improving moisture tolerance, thereby enabling superior sensitivity under humid conditions. Transition-metal dopants, whose ionic radii are often comparable to those of host cations, can be readily incorporated into the lattice, significantly enhancing sensitivity by modulating carrier concentration, defect chemistry, and Ov. Non-metal dopants primarily influence surface electronic states and local electron distribution, offering notable potential for reducing operating temperatures by facilitating surface reactions and charge transfer. Future research should deepen the mechanistic understanding of the coupled dopant-host-target gas interactions, enabling targeted doping strategies that maximize sensitivity, selectivity, and stability.
Summary of elemental doping strategies
| Dopant | Advantages | Limitations | Performance enhancement effect | Typical elements |
| Rare-earth elements | Unique 4f electronic structure, high oxygen ion mobility, strong surface alkalinity, variable valence states | Higher cost of certain elements and a narrower optimal doping concentration range | Significant enhancement of selectivity and moisture resistance, modulation of the band structure, and introduction of oxygen vacancies | Ce, La, Y, Tb, Pr |
| Transition metal elements | Excellent ionic radius matching with host MOS, rich valence states, and high catalytic activity | Excessive doping leads to phase separation, reducing stability | Effective regulation of carrier concentration, grain size and oxygen vacancies to enhance sensitivity | Co, Ni, Cr, Fe, Cu, Mn |
| Non-metal elements | Effective surface energy modulation and thermal stability enhancement | Doping efficiency and uniformity control are difficult to achieve, and the mechanism of action is relatively complex | Introducing surface active sites to promote gas molecule adsorption and dissociation, and reducing operating temperature | C, N, S, Si |
Noble metal functionalization
The application of noble metals as catalysts is a well-established strategy for enhancing the functional performance of metal oxide materials. For MOS-based gas sensors, noble metal modification, typically with Pt, Pd, Au, or Ag, markedly improves sensing characteristics through two primary mechanisms: electronic sensitization and chemical sensitization[21,159,160]. As illustrated in Figure 8A, electronic sensitization arises from work function differences between the noble metal and the MOS. Charge transfer occurs until their Fermi levels align, forming a Schottky barrier and widening the EDL. This suppresses electron-hole recombination and amplifies resistance variations upon gas exposure, thereby enhancing sensor response. Chemical sensitization [Figure 8B], also known as the spillover effect, involves the dissociation of oxygen molecules on noble metal NPs, generating reactive chemisorbed oxygen species that migrate to the MOS surface. These species react rapidly with target gases, causing pronounced resistance changes. The catalytic activity of noble metals further lowers the activation energy of surface reactions, enabling lower operating temperatures and faster response-recovery dynamics.
Figure 8. (A and B) Schematic illustration of the electronic sensitization effect (A) and chemical sensitization effect (B) in n-type MOS modified with noble metals. Figure A and B is quoted with permission from Zhu et al.[21]; (C) Schematic illustration of the oxygen adsorption process (C1) and the CO sensing mechanism (C2) in mWO3 and Pt-loaded mWO3. Figure 8C is quoted with permission from Ma et al.[74]; (D) Pt NPs modified Si/WO3 nanowire array sensor and its ethanol sensing performance. Figure 8D is quoted with permission from Ren et al.[161]. NP: Nanoparticle.
In recent years, considerable efforts have been devoted to integrating porous MOS sensing materials with noble metal catalysts to construct high-performance gas sensors. Ma et al. synthesized Pt NP-sensitized mWO3 via a co-assembly strategy, exhibiting excellent catalytic sensing response toward CO at low operating temperatures[74]. Uniformly dispersed Pt NPs promoted CO oxidation and enhanced oxygen adsorption-desorption, resulting in significantly higher surface oxygen species compared with pristine mWO3, primarily due to the spillover effect [Figure 8C][74]. Moreover, Pt NPs acted as electron sensitizers, dynamically switching oxidation states during gas adsorption and desorption. Upon air exposure, the p-n heterojunction at the PtOx/WO3 interface broadened the depletion region and increased baseline resistance. CO exposure reduced PtOx to metallic Pt, eliminated the heterojunction, and injected electrons into WO3, effectively modulating the surface depletion layer. Ren et al. prepared Pt NP-modified Si-doped WO3 nanowire arrays using a one-step multicomponent co-assembly method[161]. Benefiting from the high surface area, abundant Ov, and catalytic sensitization of Pt NPs, the composite exhibited outstanding ethanol sensing at 100 °C, with high sensitivity (Ra/Rg = 93 at 50 ppm), a low detection limit (0.5 ppm), rapid response/recovery (17 s/7 s), excellent selectivity, and long-term stability [Figure 8D].
The unfilled d orbitals of noble metals enable efficient adsorption and dissociation of target gas molecules. Wang et al. employed a nano-casting method to prepare Pt-loaded mWO3 for NH3 sensing. Uniformly dispersed Pt NPs catalyzed oxygen and ammonia adsorption/desorption and promoted N-H bond cleavage[162]. Liu et al. synthesized Pd single-atom catalysts on In2O3 using a MOF templating approach, achieving higher sensitivity (6.728 ppm-1), improved H2S selectivity, and lower operating temperature and detection limit (100 ppb)[163]. DFT calculations revealed that Pd atomic sites substantially increase H2S adsorption capacity and electron transfer from adsorbed molecules. The H-S bond is readily deprotonated on Pd sites, generating S species, which were confirmed by quasi in situ X-ray photoelectron spectroscopy (XPS).
Noble metal modification also tunes the surface reaction characteristics of MOS, thereby controlling gas selectivity. Kim et al. used hollow apoferritin (AF) as a template to fabricate mWO3 NFs decorated with Pt-AF, Pd-AF, or Rh-AF NPs. These sensors exhibited selective responses to 1 ppm acetone (Pt-AF_WO3 NFs: Ra/Rg = 62), toluene (Pd-AF_WO3 NFs: Ra/Rg = 11), and H2S (Rh-AF_WO3 NFs: Ra/Rg = 21)[164]. More recently, Park et al. reported Ru, Pd, and Pt modified In2O3 NFs to systematically investigate noble metal-induced selectivity[165]. At 250 °C, Ru-In2O3, Pd-In2O3, and Pt-In2O3 exhibited distinct selectivity toward CH3SH, H2S, and CH3COCH3, respectively. Highly dispersed noble metal species promote oxygen chemisorption through spillover, creating abundant active sites for gas reactions. DFT and Bader charge analyses showed that selectivity originates from differences in adsorption energies and charge transfer between target gases and metal atoms. Ru and Pd selectively differentiate CH3SH and H2S based on adsorption energies, whereas Pt primarily governs selectivity by facilitating charge transfer with CH3COCH3.
Beyond single-metal catalysts, bimetallic NPs have been employed to further enhance and tailor the sensing properties of MOS. Sui et al. functionalized monodisperse AuPt NPs (~ 9 nm) onto In2O3 NFs to construct a highly efficient gas sensor[166]. This device achieved dual selectivity toward O3 and C3H6O at different optimal temperatures, with ppb-level detection limits. The performance enhancement arises from the synergistic catalytic effects of AuPt NPs: (1) the d-band center shifts endow AuPt with high electrocatalytic activity; (2) chemical sensitization promotes O2 dissociation into adsorbed oxygen (Oads); and (3) the Schottky barrier at the In2O3/AuPt interface broadens the electric double layer, improving carrier transport during sensing. Xue et al. reported mesoporous SnO2 microspheres functionalized with Pt-Pd nanoalloys (Pt-Pd/SnO2)[6]. The confined nanoalloys induce Ov within SnO2, increasing carrier concentration. Among them, Pt1-Pd4/SnO2 exhibited exceptional CH4 sensing performance, with a high response (Ra/Rg = 21.33 at 3,000 ppm), fast response/recovery times (4 s/9 s), and excellent stability. DFT calculations revealed that covalent bonding between the Pt-Pd nanoalloy and SnO2, combined with rapid electron transfer, promotes orbital hybridization between Pd4 sites and adsorbed CH4, thereby enhancing CH4 adsorption and catalytic activation.
The functionalization of noble metals provides a highly effective pathway to enhance the gas sensing performance of MOS materials through the combined contributions of electronic and chemical sensitization. Owing to their distinct electronic configurations and catalytic activities, different noble metals play distinct functional roles and exhibit specific application preferences. Pt couples exceptional catalytic activity for oxygen dissociation with strong electronic interactions, enabling ultrahigh sensitivity and rapid response toward reductive small molecules such as CO and H2. Pd is indispensable for hydrogen sensing due to its unique ability to adsorb and dissociate H2, producing a pronounced resistance change associated with the Pd/PdHx phase transition. However, its susceptibility to poisoning remains a performance-limiting factor. The performance enhancement induced by Au is strongly dependent on its size and morphology. Ultrafine Au NPs can activate low-temperature or even room-temperature sensing through synergistic effects between surface plasmon resonance (SPR)-induced charge excitation and catalytic oxidation. Ag, a more cost-effective noble metal, has demonstrated remarkable selectivity toward sulfur-containing gases owing to its favorable adsorption and catalytic oxidation characteristics toward S-H or S-based functional groups. Overall, effective noble-metal functionalization relies on identifying catalytic sites capable of activating specific chemical bonds in target gas molecules (e.g., C-H, H-H, S-H) and simultaneously promoting significant interfacial charge transfer. Maximizing these synergistic effects further depends on precise control over noble-metal particle size, dispersion, oxidation state, and metal-support interactions.
Heterojunction engineering
Forming heterojunctions by combining different MOS materials is a widely adopted strategy to enhance gas sensor performance. A heterojunction is the physical interface between two distinct semiconductors. When they come into contact, the difference in Fermi levels drives charge transfer until equilibrium is reached, creating depletion layers and potential barriers at the interface[167]. These interfacial features markedly improve gas sensing responses. Porous semiconductors, with their large surface areas and abundant interfaces, are particularly advantageous for constructing multiple heterojunctions, thereby enabling high sensitivity and selectivity. Optimizing material combinations and heterojunction architectures can further boost sensor performance in practical applications. Depending on the carrier type of the constituent materials, three primary heterojunction types can be formed, namely p-n, n-n, and p-p junctions.
p-n junctions
The p-n junction is widely regarded as the most effective heterostructure for gas sensors because the work function difference between the two MOS components drives electron transfer from the higher Fermi level to that with a lower Fermi level. Upon heterojunction formation, Fermi level mismatch further induces charge redistribution, thereby significantly modifying the electronic structure of the nanocomposite. As illustrated in Figure 9A, electrons migrate from the n-type to the p-type MOS, while holes move in the opposite direction until the Fermi level equilibrium is reached[46]. This process generates a space-charge layer and band bending on both sides of the interface, creating a potential barrier that narrows the electron transport channel. Oxygen adsorption suppresses electron transfer and reduces the effective cross-section for charge conduction, thereby increasing resistance. Exposure to reducing gases triggers reactions with adsorbed oxygen species, releasing electrons back into the MOS and lowering resistance. The large resistance changes before and after gas exposure enhance sensing performance.
Figure 9. (A) Schematic illustration of the band structure of a p-n heterojunction before and after contact. Figure 9A is quoted with permission from Mondal et al.[46]; (B) Schematic illustration of the band structure (B1) and surface oxygen vacancy sites (B2) of the In2O3/NiO heterostructure. Figure 9B is quoted with permission from Xie et al.[169]; (C) NOx gas sensing mechanism on ordered mesoporous WO3/ZnO n-n heterojunction. Figure 9C is quoted with permission from Han et al.[174]; (D) NH3 gas sensing mechanism on CuO/α-Fe2O3 p-p heterojunction. Figure 9D is quoted with permission from Bhuvaneshwari et al.[175]; (E) Synthesis route diagram (E1) and ethanol sensing performance (E2) of multilevel dendritic mesoporous TiO2-SnO2 composites. Figure 9E is quoted with permission from Zhao et al.[76]. PEO: Poly(ethylene oxide).
Wang et al. reported a highly selective and sensitive 3H-2B sensor based on Co3O4 NP-modified 3D radial ZnO nanorods[168]. The ZnO/Co3O4 composite exhibited ultrahigh sensitivity (Ra/Rg = 550 at 5 ppm), an ultralow detection limit (10 ppb), and excellent selectivity at 260 °C, attributed to the p-n heterojunction modulated depletion layer and the radial structure that facilitates gas diffusion. The p-n junction also provides additional Ov and active adsorption sites, further improving sensing performance. For example, Xie et al. fabricated a NiO-functionalized 3D ordered macroporous In2O3 thin-film sensor via atomic layer deposition (ALD)[169]. This approach precisely controlled Ov concentration and formed p-n heterojunctions within the In2O3/NiO composite, effectively tuning surface chemistry and electrical properties [Figure 9B]. The optimized sensor exhibited a response of 532.2 to 10 ppm NO₂ at 145 °C, representing a 26-fold improvement over pristine In2O3.
Surface reactions at p-n heterojunction interfaces also play a critical role in achieving high sensing performance. Xiao et al. fabricated mesoporous p-n heterojunction MOS based on n-type WO3 and p-type NiO[170]. Oxygen adsorption creates an electron-depleted surface layer before H2S exposure. Upon contact with H2S, both adsorbed oxygen and metal oxides react with the gas, releasing abundant electrons and sharply decreasing resistance. During sensing, SO2 and conductive sulfides (WS2 and NiS) are formed, further reducing resistance and enhancing performance. The mesoporous structure, with high porosity and abundant active sites, facilitates gas diffusion and adsorption. At the same time, ultrafine NiO nanocrystals embedded in the WO3 framework provide numerous p-n junctions, thereby collectively yielding outstanding H2S sensing performance. Similarly, Shao et al. demonstrated that decorating n-type SnO2 nanowires with p-type CuO NPs significantly improves H2S detection[171]. In air, the extended EDL induced by CuO NPs increases resistance, whereas exposure to H2S leads to the formation of CuS, which eliminates nanoscale p–n junctions and results in a sharp resistance drop.
Heterojunctions also influence selectivity. Jeong et al. converted Co3O4 HSs into Co3O4-SnO2 core-shell HSs via electrochemical replacement[172]. While pure Co3O4 showed selectivity toward ethanol, the core-shell structures exhibited high selectivity to xylene and toluene at 275 °C and 300 °C, respectively, owing to the synergistic catalytic effects of SnO2 and Co3O4 combined with the pore confinement effect. Moreover, the microstructure and exposure configuration of heterojunctions are crucial. Woo et al. investigated ZnO-Cr2O3 nanowire heterojunctions for TMA detection[173]. They found that decoration with discrete Cr2O3 NPs enhanced the response through catalytic activity and p-n junction formation, whereas uniform Cr2O3 shell coating led to p-type conduction dominance and diminished response compared to pure ZnO nanowires.
n-n or p-p junctions
Band bending can also occur in n-n and p-p heterojunctions due to energy level differences between two n-type (or p-type) metal oxides. For n-n heterojunctions, variations in conduction band energies drive electron transfer across the interface, leading to depletion in the material with the higher conduction band energy and accumulation in that with the lower energy [Figure 9C][174]. In p-p heterojunctions, holes serve as the majority carriers [Figure 9D][175]. Differences in valence band energies cause holes to migrate from the material with the higher valence band energy to the one with lower energy, resulting in hole depletion and accumulation regions, respectively[176]. Both n-n and p-p junctions arise from work function differences, which induce carrier migration to maintain equilibrium and amplify electrical signal variations[30]. Additionally, heterojunction formation promotes oxygen adsorption, generating abundant Ov and providing extra active sites for sensing reactions[177]. The mesoporous structure of MOS composites further facilitates gas adsorption and desorption, collectively enhancing sensor sensitivity and response speed.
Zhao et al. constructed multi-scale dendritic mesoporous TiO2-SnO2 nanocomposites with well-defined n-n heterojunctions for highly sensitive ethanol detection [Figure 9E][76]. The hydroxyl-rich surface of SnO2 nanocrystals forms hydrogen bonds with the PEO segments of the PEO-b-PS template, enabling their homogeneous dispersion within the mesoporous TiO2 matrix and generating numerous TiO2-SnO2 n-n heterojunctions. Electron transfer from TiO2 to SnO2 leads to surface electron accumulation on SnO2, which is subsequently depleted by oxygen adsorption, increasing the interfacial energy barrier and enhancing the gas response. Additionally, the multi-level dendritic mesoporous structure, featuring large pores, abundant active sites, and short diffusion paths, accelerates gas diffusion and reaction, thereby improving sensitivity and response speed. Similarly, Wang et al. synthesized highly crystalline mesoporous TiO2/WO3 n-n heterojunctions via an acid-base pair-regulated self-assembly route[178]. The material exhibits large pores (~ 21.1 nm), crystalline frameworks, and high surface area, enabling efficient oxygen adsorption and rapid acetone diffusion. Importantly, the TiO2/WO3 n-n heterojunction promotes the formation of an EDL, thus amplifying the sensing response and ensuring fast response/recovery characteristics. Other n-n heterojunction systems, including SnO2-ZnO hollow dodecahedra[179], porous MO3/SnO2 NSs[180], In2O3@SnO2 core-shell NFs[181], and 3D anti-opalescent ZnO-In2O3 multilayer films[182], have also demonstrated significantly enhanced gas sensing performance.
The formation of p-p heterojunctions can also markedly enhance gas sensing performance. Alali et al. fabricated porous Co3O4/CuO p-p heterojunction nanotubes by electrospinning and demonstrated that visible light irradiation significantly improves their response to dimethyl methylphosphonate (DMMP), a simulant of sarin nerve agent[77]. The Co3O4-CuO heterojunction lowers the interfacial potential barrier, and oxygen chemisorption increases hole concentration, leading to reduced resistance in air. Light excitation further promotes the generation of holes by transferring electrons from the valence to the conduction band, thereby decreasing resistance. In a reducing DMMP atmosphere, electron donation to the sensing layer results in a pronounced resistance increase. Suh et al. synthesized NiO-modified Co3O4 nanorods via multi-step grazing-incidence deposition for efficient benzene detection[183]. The conformal NiO coating induces numerous p-p heterojunctions, lowering hole concentrations in Co3O4 and thus increasing baseline resistance, which amplifies the sensing response compared to pristine Co3O4. Additionally, modified surface lattice orientation and the catalytic activity of NiO further enhance gas sensing properties. Other p-p heterojunction systems, including mesoporous NiO@CuO flower-like hierarchical composites[184], Cu2O/CuO core-shell nanowires[185], and NiO/NiCr2O4 nanocomposites[186], have also demonstrated significantly improved sensing performance.
Overall, heterojunction engineering provides a versatile and powerful toolbox for tailoring the electronic and chemical properties of MOS sensing materials. By carefully selecting material pairs and controlling interface nanostructure, it is possible to achieve unprecedented levels of sensitivity, selectivity, and response speed. The design of high-performance heterojunction-based MOS sensors relies on several fundamental principles. The band alignment between two semiconductors determines the direction and degree of interfacial charge transfer, which directly modulates the width of the depletion layer and the height of the potential barrier. The presence of mesoporous frameworks can amplify interfacial effects by providing abundant exposed junctions and facilitating rapid gas diffusion. The selection of semiconductor components with complementary catalytic activities and adsorption affinities enables synergistic surface reactions. These principles collectively provide a rational foundation for engineering heterojunctions with optimized electronic structures, enhanced reaction kinetics, and improved sensing performance. The following section on applications will showcase how these designed heterojunctions address specific detection challenges across various fields.
APPLICATION SCENARIOS
Industrial and public safety
Gas sensors are widely employed in daily life and industrial production for detecting toxic, hazardous, flammable, and explosive gases such as methane, hydrogen, carbon monoxide (CO), and ethanol.
Methane (CH4), the principal component of natural and coal gas, is extensively used as a household and industrial fuel as well as a key feedstock for producing carbon black, acetylene, carbon disulfide, and methanol. However, CH4 is also the major constituent of coal mine gas. When mixed with air at volume fractions between 4.9% and 15.4%[187], it forms highly explosive mixtures that pose severe safety risks during coal mining operations. Therefore, rapid and real-time CH4 detection is critical for both industrial safety and domestic applications. Recently, Xue et al. reported a CH4 sensor based on Pt-Pd nanoalloy modified mesoporous SnO2 microspheres, which exhibited high sensitivity (Ra/Rg = 21.33), fast response/recovery (4 s/9 s), and excellent reversibility toward 3000 ppm CH4 at 402 °C[6]. This sensor can be readily integrated into portable wireless modules, enabling real-time CH4 monitoring via smartphones. Other multicomponent MOS sensors reported for CH4 detection include ZnO/NiO[187], ZnO/SnO2[188], Pd-SnO2[189], and Pt-SnO2/ZnO[190].
Hydrogen (H2) is considered one of the most promising clean energy sources due to its abundance and the fact that its combustion produces only environmentally benign water (H2O). However, H2 is highly flammable with a lower flammability limit of 4%, and its colorless, odorless, and buoyant nature makes leaks difficult to detect. Therefore, developing efficient and sensitive H2 sensors is essential for rapid leak detection and timely safety warnings. Zhang et al. synthesized Pd NP-modified Si-doped mWO3 microspheres (Pd/Si-mWO3), which exhibited excellent H2 selectivity, high sensitivity (Ra/Rg = 33.5 at 50 ppm H2), and good moisture resistance [Figure 10A][5]. Other multicomponent MOS-based H2 sensors have been reported, including SnO2-ZnO[191], NiO/TiO2[192], Pd-SnO2[193], and PdAg/SnO2[194].
Figure 10. (A) Pd/Si-mWO3 microspheres-based H2 gas sensing. SEM images of Si-mWO3 (A1); Sensing response to 50 ppm different gases (A2); Response curves toward various H2 concentrations at 210 °C (A3). Figure 10A is quoted with permission from Zhang et al.[5]; (B) TiOx-In2O3-based NO2 gas sensing. Schematic illustration of gas sensing mechanism under light activation (B1); cyclic performance of 5TiOx-In2O3 against 1 ppm NO2 (B2); average response values toward 3 ppm NO2 at dry, 20%, 50%, and 80% RH conditions (B3). Figure 10B is quoted with permission from Park et al.[201]; (C) WO3/Au-based 3-hydroxy-2-butanone gas sensing. Dynamic response-recovery characteristic curves of WO3/Au-based sensors exposed to different concentrations of 3-hydroxy-2-butanone gas (C1); Scheme illustration of MOS-based gas sensors applied in the selective detection of 3-hydroxy-2-butanone (C2). Figure 10C is quoted with permission from Ma et al.[209]; (D) Au@m-WO3 spheres-based isoprene gas sensing. Schematic of a man running on a treadmill (D1). Concentrations of isoprene and gas response of the Au@m-WO3 sensor to the exhaled breath as a function of running time (D2). Figure 10D is quoted with permission from Park et al.[229]. SEM: scanning electron microscope; ppm: parts-per-million.
CO is a colorless, odorless gas with a density slightly lower than air, mainly produced by vehicle exhaust and incomplete fossil fuel combustion. Owing to its strong affinity for hemoglobin, CO inhibits oxygen transport, leading to nausea, dizziness, unconsciousness, and potentially death. In addition, CO is flammable and explosive, with lower and upper explosive limits of 12.5 vol% and 74.2 vol% in air, respectively. Therefore, the development of high-performance CO sensors featuring fast response, high selectivity, and low detection limits is of critical importance. Ma et al. synthesized mWO3 sensitized with Pt NPs via a simple co-assembly strategy[74]. The resulting composite exhibited excellent sensing performance toward 100 ppm CO at 125 °C, including ultrafast response/recovery times (16 s/1 s), high sensitivity (Ra/Rg = 10 ± 1), and outstanding selectivity. Subsequently, Ma et al. prepared a mesoporous SiO2/WO3-x composite decorated with ultrathin FeOx nanolayers selectively deposited on Pt NPs (SiO2/WO3-x-Fe2O3/Pt)[62]. This SiO2/WO3-x-Fe2O3/Pt-based sensor achieved superior performance, with higher sensitivity (Ra/Rg = 31 ± 2 at 50 ppm CO) and a lower optimal operating temperature of 66 °C. Other reported multicomponent MOS sensors for CO detection include Mn-doped Co3O4[138], CuO-SnO2[195], and PtAg/WO3[196].
Ethanol (C2H5OH) is a highly volatile and flammable compound widely used in daily life, industrial production, and healthcare. Undetected ethanol leaks can lead to severe accidents upon contact with open flames. In addition, ethanol sensors are employed for breath alcohol analysis to accurately assess drivers’ intoxication levels, playing a crucial role in traffic safety. Therefore, developing high-performance ethanol sensors is essential. Recently, our group[197] synthesized Au NP-modified ordered mesoporous SiO2-WO3 composites. Controlled etching of SiO2 from the pore walls increased the specific surface area (129 m2·g-1) and improved pore connectivity (window size > 4.3 nm). Sensors based on this material showed excellent detection of ultra-trace ethanol concentrations (ppb level), achieving high sensitivity (Ra/Rg = 2-14 at ethanol concentrations of 50-250 ppb) at low operating temperatures (150 °C). Other reported multicomponent MOS ethanol sensors include Tb-doped In2O3[129], TiO2-SnO2[76], ZnO-Au[198], and Pt/Si-WO3[161].
Environmental monitoring
Nitrogen dioxide (NO2) is one of the most harmful atmospheric pollutants, mainly emitted from fossil fuel combustion, motor vehicle exhaust, and industrial processes. In the atmosphere, NO2 forms nitric acid aerosols, contributing significantly to acid rain and photochemical smog. Beyond its environmental impact, NO2 poses serious health risks. Even low concentrations can damage the respiratory system and lung tissue and aggravate conditions affecting the eyes, nose, and throat. According to the US National Institute for Occupational Safety and Health (NIOSH), the recommended exposure limit for NO2 is 1 ppm. Therefore, the development of sensors capable of rapidly and accurately detecting trace NO2 concentrations is essential for the protection of both environmental and human health. Reported NO2 sensors based on multicomponent MOS materials include Ni-doped In2O3[142], S-doped SnO2[157], In2O3/NiO[169], NiO@CuO[184], Pt/SnO2[199], and Au@ZnO/reduced graphene oxide (rGO)[200]. For example, Park et al. designed a high-performance NO2 sensor by constructing a heterojunction between photoactive TiOx and In2O3 layers [Figure 10B][201]. The TiOx coating modulated the chemical and electronic properties of the In2O3 sensing layer and provided additional NO2 adsorption sites. Under ultraviolet illumination, this heterojunction exhibited a markedly enhanced sensing response (|ΔR/R0| = 35.8 at 5 ppm).
Formaldehyde (HCHO) is a colorless gas that is challenging to detect at low concentrations yet emits a strong, pungent odor at higher levels. It is widely used industrially as a preservative, disinfectant, and germicide, and it also ranks among the most common indoor air pollutants, often originating from low-quality furniture and building materials. Prolonged exposure to elevated HCHO concentrations can cause dizziness, headaches, watery eyes, nausea, coughing, chest tightness, and leukemia, and may even be fatal in severe cases. In addition, HCHO exhibits chronic toxicity, including genotoxic, carcinogenic, and teratogenic effects, leading to its classification as a Group 1 carcinogen. The occupational exposure limits set by the NIOSH and the World Health Organization (WHO) are 1 ppm and 0.08 ppm, respectively. Therefore, the development of sensors capable of rapidly and accurately detecting trace indoor HCHO is of great importance. Reported multicomponent MOS-based HCHO sensors include Y-doped In2O3[128], Co-doped SnO2[141], CuO/SnO2[27], In2O3@SnO2[181], Ag-SnO2[60], and Au25/In2O3[202], among others.
Benzene, toluene, ethylbenzene, and xylene (collectively known as BTEX) are highly toxic and carcinogenic volatile organic compounds (VOCs). Major BTEX emissions originate from crude oil refining and coal combustion, followed by industrial activities such as paint, adhesive, and pesticide production, as well as transportation and product use. These compounds not only contaminate the environment but also pose serious health risks. Short-term exposure may cause dizziness, headaches, and irritation of the eyes, throat, and skin, whereas chronic exposure can damage the nervous and respiratory systems. Benzene and ethylbenzene are associated with bone marrow disorders and leukemia, xylene may impair kidney and liver function, and toluene can induce pregnancy termination. Accordingly, the development of high-performance BTEX sensors is essential for environmental and health protection. Hu et al. fabricated a Co-doped g-C3N4/ZnO nanocomposite sensor and systematically evaluated its BTEX sensing behavior[203]. At an operating temperature of 370 °C, the sensor showed markedly enhanced responsiveness to o-, m-, and p-xylene compared with other gases, with approximately 11-fold higher response intensity than pure ZnO. It also demonstrated excellent stability, repeatability, and rapid response/recovery times (2 s/2 s). Other multicomponent MOS sensors for BTEX detection include Ti-doped Co3O4[204], Rh-TiO2/SnO2[205], ZnO-CeO2[206], and CuO/WO3[28].
TEA is a colorless, highly volatile liquid with a strong ammonia-like odor, widely used in agriculture, fisheries, chemical industries, and medical applications. It serves as an organic solvent, polymerization inhibitor, preservative, and catalyst, and is also used in the treatment of hypertension, angina pectoris, variant angina, and chronic stable angina. In addition, TEA is released from spoiled fish and seafood, allowing it to function as an indicator of marine product freshness. However, excessive TEA exposure can severely damage the skin, mucous membranes, liver, circulatory system, and central nervous system, and prolonged exposure may cause emphysema or even death. According to NIOSH, the recommended indoor concentration limit for TEA is 10 ppm. Therefore, the development of high-performance sensors capable of rapid, real-time TEA detection is of great importance. Zhou et al. synthesized a Pt-supported Ce-doped In2O3 composite, which exhibited excellent TEA selectivity, ultra-high sensitivity (Ra/Rg = 1,110 at 10 ppm), and good long-term stability[207]. Other reported multicomponent MOS sensors for TEA detection include Co3O4/In2O3[75], La2O3-In2O3[107], Mo-doped Co3O4[146], Fe-doped NiO[139], and Ag-WO3[208].
Food quality assessment
Volatile compounds not only impart distinctive flavors and aromas to foods but also serve as important indicators of spoilage, contamination, and hazardous substances. The odors of food ingredients contain key information about their condition, making gas sensors essential for food quality control, freshness evaluation, and safety monitoring. In the food industry, MOS gas sensors have emerged as highly effective detection devices due to their high sensitivity, rapid response, miniaturization, low cost, and durability. Continued research and technological advances have further improved their performance, reinforcing their role in ensuring food quality and freshness.
Listeria monocytogenes (LM) is a highly pathogenic foodborne bacterium found in vegetables, fish, and dairy products. Even low levels of contamination can cause serious illnesses such as septicemia and meningitis, particularly in immunocompromised individuals. Conventional LM detection methods are typically slow, labor-intensive, and costly, limiting their practical use. Recent studies have identified 3H-2B as a volatile biomarker for LM, enabling rapid and convenient screening via MOS gas sensors. Our group synthesized a series of ordered dual mesoporous TMO/NM nanocomposites (TMO = WO3, TiO2, NbOx; NM = Au, Pd, AuPd) through a co-assembly strategy mediated by soluble thiophenol-formaldehyde resin [Figure 10C][209]. Benefiting from noble metal sensitization and the dual mesoporous structure, WO3/Au-based sensors exhibited outstanding 3H-2B sensing performance, including high sensitivity (Ra/Rg = 18.8 at 2.5 ppm), high selectivity (≥ 4-fold over interfering gases), rapid response/recovery times (15/45 s at 2.5 ppm), and an ultra-low detection limit (50 ppb) at a low operating temperature (175 °C).
Furthermore, the sensor can be integrated into compact wireless modules for real-time monitoring and display of 3H-2B levels on smartphones, demonstrating strong potential for rapid LM detection. Other multicomponent MOS sensing materials reported for 3H-2B detection include P-doped mWO3[63], Tb-doped WO3[210], ZnO/Co3O4[168], CeO2/SnO2[211], PtCu/WO3[212], and AuPd-WO3[213].
Ammonia (NH3) is a colorless, toxic gas with a pungent odor. In daily life, it primarily arises from the spoilage of high-protein foods such as eggs, meat, and dairy products, where microbial and enzymatic degradation of proteins releases NH3. Consequently, NH3 concentration serves as a key indicator for assessing the freshness of protein-rich foods. Reported NH3 sensors based on multicomponent MOS materials include Cr-doped In2O3[136], Si-doped α-MoO3[155], Pt-WO3[162], and ZnO/SnO2[214]. For example, Ajjaq et al. hydrothermally synthesized La-doped ZnO, yielding sensors with a high response (Ra/Rg = 91) to 50 ppm NH3 at a relatively low operating temperature (150 °C), as well as excellent baseline stability and humidity resistance[215].
TMA is a colorless gas with a strong fishy odor, typically produced during the spoilage of fish and seafood. As storage time increases and freshness declines, TMA oxide in aquatic products is gradually reduced or thermally decomposed into TMA, leading to a marked increase in TMA concentration. TMA levels below 10 ppm indicate freshness, 10-50 ppm suggest initial spoilage, and > 60 ppm signify severe deterioration[216]. Therefore, TMA concentration is widely regarded as a critical indicator of seafood freshness. Monitoring TMA levels enables effective quality assessment and ensures food safety. Shen et al. developed a TMA sensor based on α-Fe2O3 hollow nanocubes modified with bimetallic Au@Pt nanocrystals, which exhibited a rapid response (5 s), high sensitivity (Ra/Rg = 32 at 100 ppm), and excellent selectivity at 150 °C[217]. The sensor also demonstrated high responsiveness to trace TMA concentrations in both standard and real gas samples released from the large yellow croaker. Other reported multicomponent MOS sensors for TMA detection include Au-WO3[216], Pr-doped Ce4W9O33[218], Ce-doped MoO3[131], ZnO-Cr2O3[173], and NiO-In2O3[219].
Disease diagnosis
Recent studies have revealed that various molecules in exhaled breath are strongly correlated with specific diseases and metabolic processes. For example, acetone, NO2, NH3, H2S, and certain VOCs are recognized as biomarkers for diabetes, asthma, kidney disease, halitosis, and lung cancer, respectively. By analyzing the composition and concentration of these biomarkers, non-invasive disease diagnosis can be achieved. Breath analysis offers distinct advantages, including low cost, simplicity, and patient comfort, making it a promising clinical diagnostic tool.
Acetone (C3H6O) is a well-established biomarker for diabetes, exhibiting markedly different concentrations in healthy and diseased individuals. Clinical data indicate that acetone levels in exhaled breath typically range from 300 to 900 ppb in healthy subjects, whereas diabetic patients often exhibit concentrations above 1.8 ppm[220].
Consequently, breath acetone levels can serve as a reliable basis for diabetes diagnosis. In recent years, non-invasive diagnostics based on MOS sensors have attracted increasing attention due to their high sensitivity, rapid response, low cost, and facile miniaturization, offering significant application potential. For example, Verma et al. synthesized Pd/WO3 with a hierarchical nanostructure that exhibited excellent acetone sensitivity, with a response of 8.22 at 1 ppm and 57.33 at 100 ppm[221]. To demonstrate practical applicability, the sensor was integrated into a cellulose paper mask for real-time breath analysis, yielding a response of 1.70 at 8 vol% (equivalent to a single exhalation). Other reported multicomponent mesoporous MOS sensing materials for acetone detection include ZnO-SiO2[100], Si-doped WO3[153], CeO2-WO3[220], Tb4O7-In2O3[222], ZnO/Co3O4[223], Pt-SnO2[224], and AuPt-In2O3[166].
Hydrogen sulfide (H2S) is a characteristic biomarker for oral diseases such as tongue coating, periodontal disease, and mucosal disorders, originating from bacterial metabolism of organic matter. Clinical studies have shown that the average H2S concentration in the breath of halitosis patients (20.64 ppb) is markedly higher than that of healthy individuals (3.36 ppb), indicating its potential as a non-invasive diagnostic marker for halitosis[225]. H2S is also an early-stage biomarker for meat spoilage caused by bacterial growth[226]. Therefore, accurate ppb-level H2S monitoring is essential for applications in both healthcare and food safety. Additionally, due to its toxicity, prolonged exposure to concentrations above 10 ppm can cause severe health effects, necessitating continuous monitoring at higher concentration levels. Reported multicomponent MOS sensors for H2S detection include M-doped SnO2 (M = Cr, Mo, W)[227], WO3/NiO[170], In2O3/ZnO[110], NiO-CuO[111], In2O3/Pdatom[163], and Pt-WO3[228].
Isoprene (C5H8) is a representative exhaled gas produced during cholesterol biosynthesis and muscle metabolism. Its concentration in the breath of hyperlipidemic patients is typically higher than that of healthy individuals (~ 100 ppb), and it rises significantly during physical exercise. Therefore, precise monitoring of C5H8 levels provides valuable information for assessing physiological states and enabling early diagnosis of cardiovascular diseases. Park et al. developed a highly selective and sensitive gas sensor based on macroporous WO3 microspheres encapsulated with Au NPs [Figure 10D][229]. This sensor exhibited high responsivity (Ra/Rg = 11.3) and an extremely low detection limit (0.2 ppb) towards 0.1 ppm C5H8 under high humidity at 275 °C. Similarly, Park et al.[8] employed a ZIF-67 template to synthesize yolk-shell Co3O4@CoPW structures comprising a catalytic Co3O4 core and a semi-permeable polyoxometalate shell. This architecture prolongs gas residence time and promotes intermediate regeneration, resulting in exceptional C5H8 selectivity with a high response (180.6 vs. 5 ppm) and a low detection limit (0.58 ppb).
CONCLUSION AND OUTLOOK
This review summarizes recent advances in the design strategies and practical applications of multicomponent porous MOS gas sensors. Key sensing mechanisms, performance evaluation parameters, and structural factors influencing sensor behavior are outlined. Representative strategies for performance enhancement - such as mesopore construction, elemental doping, noble metal loading, and heterojunction engineering - are discussed. Their broad applications in industrial safety, environmental monitoring, food quality assessment, and non-invasive disease diagnosis are also highlighted.
Looking forward, the development of MOS gas sensors is expected to advance toward integrated, intelligent, and scalable systems, driven by both materials innovation and technological convergence. From a materials perspective, multicomponent integration and hierarchical structural design will remain central to performance optimization. High-throughput synthesis combined with machine learning assisted screening will significantly accelerate the discovery of novel classes of materials, including high-entropy oxides, MOF-derived composites, and multi-heterojunction frameworks, enabling precise structural tuning and property optimization.
Equally important is the transition toward low-temperature or room-temperature sensing, which is crucial for flexible electronics and portable miniaturized devices. Achieving this requires the synergistic use of plasmonic or catalytic sensitization, interfacial band engineering, and light-assisted activation to lower reaction barriers, enhance charge separation, and improve energy efficiency. These approaches will support the development of wearable, low-power, and continuously operating sensing platforms.
A particularly promising direction lies in smart and multifunctional sensing. Future sensors will move beyond single-parameter detection toward multidimensional information fusion and intelligent perception. By integrating sensor arrays with AI algorithms, machine learning, and deep neural networks, selective recognition of complex gas mixtures, adaptive calibration, and real-time data analysis can be achieved. This transformation will enable practical deployment in smart homes, healthcare monitoring, environmental networks, and other IoT-embedded applications.
Despite these exciting prospects, several industrial and technological bottlenecks must be addressed before multicomponent porous MOS sensors can achieve large-scale commercial deployment. First, environmental adaptability and long-term stability remain major obstacles. Realistic conditions, such as humidity interference and cross-interaction of multi-component gases, often induce baseline drift and degrade sensitivity. Developing humidity-tolerant components, hydrophobic surface modification, self-regenerative catalytic interfaces, and in situ monitoring of sensor drift will be essential. Second, the scalability and cost-effectiveness of synthesis routes present practical barriers. Many high-performance multicomponent MOS structures rely on complex templating, high-temperature calcination, or noble-metal catalysts, thereby increasing manufacturing costs. To overcome these limitations, future efforts should focus on template-free mesoscale structuring, earth-abundant catalytic alternatives, and low-temperature crystallization. Third, integration with microfabrication and heterogeneous electronics remains challenging. Powder-based MOS layers often exhibit poor adhesion to substrates, leading to device inconsistency. In situ growth strategies - including vapor-phase deposition, ALD, inkjet printing, and solution processing - are expected to significantly enhance device uniformity and reliability. Finally, the transition toward multi-parameter sensing and intelligent data fusion requires advances in sensor arrays, selective interfaces, and machine-learning-assisted pattern recognition. Addressing these challenges through materials innovation, scalable manufacturing, and intelligent integration will be key to bridging the gap between laboratory demonstrations and real-world implementation.
In summary, the future of MOS gas sensing will center on multicomponent synergy, structural precision, intelligent functionality, and scalable integration, while tackling pressing challenges in selectivity, stability, mechanistic understanding, and device manufacturing. Continued interdisciplinary efforts across materials science, device engineering, data science, and microfabrication will be pivotal in realizing the next generation of intelligent, miniaturized, and application-ready gas sensing technologies.
DECLARATIONS
Authors’ contributions
Conception and manuscript revision: Deng, Y.
Manuscript writing: Xue, L.; Li, Y.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (Nos. 22125501, U22A20152) and the Science and Technology Commission of Shanghai Municipality (No. 2024ZDSYS02).
Conflicts of interest
Deng, Y. is the Editor-in-Chief of the journal Micro Nano Science. Deng, Y. was not involved in any steps of the editorial process, including reviewers’ selection, manuscript handling, or decision-making. The other authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. He, X.; Chai, H.; Luo, Y.; Min, L.; Debliquy, M.; Zhang, C. Metal oxide semiconductor gas sensing materials for early lung cancer diagnosis. J. Adv. Ceram. 2023, 12, 207-27.
2. Xue, L.; Cui, J.; Li, R.; et al. Interface engineering p-n heterostructured core-shell mesoporous particles for cascade catalysis promoted gas sensing. Adv. Mater. 2025, 37, e2416006.
3. Hooshmand, S.; Kassanos, P.; Keshavarz, M.; et al. Wearable nano-based gas sensors for environmental monitoring and encountered challenges in optimization. Sensors. (Basel). 2023, 23, 8648.
4. Wawrzyniak, J. Advancements in improving selectivity of metal oxide semiconductor gas sensors opening new perspectives for their application in food industry. Sensors. (Basel). 2023, 23, 9548.
5. Zhang, Z.; Ma, J.; Deng, Y.; et al. Polymerization-induced aggregation approach toward uniform Pd nanoparticle-decorated mesoporous SiO2/WO3 microspheres for hydrogen sensing. ACS. Appl. Mater. Interfaces. 2023, 15, 15721-31.
6. Xue, L.; Ren, Y.; Li, Y.; et al. Pt-Pd nanoalloys functionalized mesoporous SnO2 spheres: tailored synthesis, sensing mechanism, and device integration. Small 2023, 19, e2302327.
7. Li, C.; Choi, P. G.; Masuda, Y. Highly sensitive and selective gas sensors based on NiO/MnO2@NiO nanosheets to detect allyl mercaptan gas released by humans under psychological stress. Adv. Sci. (Weinh). 2022, 9, e2202442.
8. Park, S. J.; Moon, Y. K.; Park, S. W.; et al. Highly sensitive and selective real-time breath isoprene detection using the gas reforming reaction of MOF-derived nanoreactors. ACS. Appl. Mater. Interfaces. 2023, 15, 7102-11.
9. Bu, W.; Zhou, Y.; Huang, D.; et al. Ppb-level unsymmetrical dimethylhydrazine detection based on In2O3 hollow microspheres with Nd doping. J. Hazard. Mater. 2024, 472, 134508.
10. Shao, X.; Zhang, D.; Zhou, L.; et al. Recent advances in semiconductor gas sensors for thermal runaway early-warning monitoring of lithium-ion batteries. Coord. Chem. Rev. 2025, 535, 216624.
11. Zhang, C.; Wang, T.; Zhang, G.; et al. Rational design and fabrication of MEMS gas sensors with long-term stability: a comprehensive review. Adv. Sci. (Weinh). 2025, 12, e11555.
12. Wang, C.; Chen, Z.; Chan, C. L. J.; et al. Biomimetic olfactory chips based on large-scale monolithically integrated nanotube sensor arrays. Nat. Electron. 2024, 7, 157-67.
13. Sung, S. H.; Suh, J. M.; Hwang, Y. J.; Jang, H. W.; Park, J. G.; Jun, S. C. Data-centric artificial olfactory system based on the eigengraph. Nat. Commun. 2024, 15, 1211.
14. Deng, Y.; Chen, K.; Luo, W.; Zou, Y.; Deng, Y. Tailoring chemiresistive nanomaterials toward integrated circuit compatible fabrication of gas sensors. Adv. Mater. 2025, e08411.
15. Yang, X.; Deng, Y.; Yang, H.; et al. Functionalization of mesoporous semiconductor metal oxides for gas sensing: recent advances and emerging challenges. Adv. Sci. (Weinh). 2022, 10, e2204810.
16. Gao, Z.; Fu, Y.; Zhang, Q.; et al. Plasmonic printing of high-performance metal oxide electronics under room temperature. Nat. Mater. 2025, 24, 2001-10.
17. Wang, C.; Song, Y.; Zhao, M.; Lu, H.; Wang, J.; Zou, X. Material design and mechanism interpretation of metal oxide nanofibers for improving gas sensitivity. Coord. Chem. Rev. 2025, 531, 216492.
18. Sivaperuman, K.; Thomas, A.; Thangavel, R.; et al. Binary and ternary metal oxide semiconductor thin films for effective gas sensing applications: a comprehensive review and future prospects. Prog. Mater. Sci. 2024, 142, 101222.
19. Sharma, A.; Eadi, S. B.; Noothalapati, H.; Otyepka, M.; Lee, H. D.; Jayaramulu, K. Porous materials as effective chemiresistive gas sensors. Chem. Soc. Rev. 2024, 53, 2530-77.
20. Wang, G.; Yang, S.; Cao, L.; et al. Engineering mesoporous semiconducting metal oxides from metal-organic frameworks for gas sensing. Coord. Chem. Rev. 2021, 445, 214086.
21. Zhu, L. Y.; Ou, L. X.; Mao, L. W.; Wu, X. Y.; Liu, Y. P.; Lu, H. L. Advances in noble metal-decorated metal oxide nanomaterials for chemiresistive gas sensors: overview. Nanomicro. Lett. 2023, 15, 89.
22. Jeong, S. Y.; Moon, Y. K.; Wang, J.; Lee, J. H. Exclusive detection of volatile aromatic hydrocarbons using bilayer oxide chemiresistors with catalytic overlayers. Nat. Commun. 2023, 14, 233.
23. Xie, W.; Huang, X.; Zhu, C.; et al. A versatile synthesis platform based on polymer cubosomes for a library of highly ordered nanoporous metal oxides particles. Adv. Mater. 2024, 36, e2313920.
24. Jiang, F.; Deng, Y.; Chen, K.; et al. A straightforward solvent-pair-enabled multicomponent coassembly approach toward noble-metal-nanoparticle-decorated mesoporous tungsten oxide for trace ammonia sensing. Adv. Mater. 2024, 36, e2313547.
25. Zhou, X.; Zhu, Y.; Luo, W.; et al. Chelation-assisted soft-template synthesis of ordered mesoporous zinc oxides for low concentration gas sensing. J. Mater. Chem. A. 2016, 4, 15064-71.
26. Yan, Y.; Lan, X.; Li, Y.; et al. Oxygen-vacancy-rich SnO2 nanoparticles based ultralow-power mems sensor for nitrogen dioxide detection. ACS. Appl. Nano. Mater. 2025, 8, 6920-9.
27. Zhu, L.; Yuan, K.; Yang, J.; et al. Fabrication of heterostructured p-CuO/n-SnO2 core-shell nanowires for enhanced sensitive and selective formaldehyde detection. Sensors. Actuat. B-Chem. 2019, 290, 233-41.
28. Guo, M.; Luo, N.; Chen, Y.; Fan, Y.; Wang, X.; Xu, J. Fast-response MEMS xylene gas sensor based on CuO/WO3 hierarchical structure. J. Hazard. Mater. 2022, 429, 127471.
29. Wang, T.; Liu, S.; Sun, P.; Wang, Y.; Shimanoe, K.; Lu, G. Unexpected and enhanced electrostatic adsorption capacity of oxygen vacancy-rich cobalt-doped In2O3 for high-sensitive MEMS toluene sensor. Sensors. Actuat. B-Chem. 2021, 342, 129949.
30. Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: a focus review. Nanoscale 2019, 11, 22664-84.
31. Ji, H.; Zeng, W.; Li, Y. Assembly of 2D nanosheets into flower-like MoO3: new insight into the petal thickness affect on gas-sensing properties. Mater. Res. Bull. 2019, 118, 110476.
32. Kim, H.; Lee, J. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sensors. Actuat. B-Chem. 2014, 192, 607-27.
33. Zhang, C.; Zhang, S.; Yang, Y.; Yu, H.; Dong, X. Highly sensitive H2S sensors based on metal-organic framework driven γ-Fe2O3 on reduced graphene oxide composites at room temperature. Sensors. Actuat. B-Chem. 2020, 325, 128804.
34. Wang, X.; Wang, Y.; Tian, F.; et al. From the surface reaction control to gas-diffusion control: the synthesis of hierarchical porous SnO2 microspheres and their gas-sensing mechanism. J. Phys. Chem. C. 2015, 119, 15963-76.
35. Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. Journal. of. Electroceramics. 2001, 7, 143-67.
36. Zhou, T.; Zhang, T. Recent progress of nanostructured sensing materials from 0D to 3D: overview of structure-property-application relationship for gas sensors. Small. Methods. 2021, 5, e2100515.
37. Panigrahi, P. K.; Chandu, B.; Puvvada, N. Recent advances in nanostructured materials for application as gas sensors. ACS. Omega. 2024, 9, 3092-122.
38. Kaur, N. Nickel oxide nanostructures for gas sensing: recent advances, challenges, and future perspectives. ACS. Sens. 2025, 10, 1641-74.
39. Wang, M.; Hou, T.; Shen, Z.; Zhao, X.; Ji, H. MOF-derived Fe2O3: Phase control and effects of phase composition on gas sensing performance. Sensors. Actuat. B-Chem. 2019, 292, 171-9.
40. Ming, J.; Wu, Y.; Wang, L.; Yu, Y.; Zhao, F. CO2-assisted template synthesis of porous hollow bi-phase γ-/α-Fe2O3 nanoparticles with high sensor property. J. Mater. Chem. 2011, 21, 17776.
41. He, J.; Rao, X.; Yang, C.; Wang, J.; Su, X.; Niu, C. Glucose-assisted synthesis of mesoporous maghemite nanoparticles with enhanced gas sensing properties. Sensors. Actuat. B-Chem. 2014, 201, 213-21.
42. Williams, D. E.; Henshaw, G. S.; Pratt, K. F. E.; Peat, R. Reaction-diffusion effects and systematic design of gas-sensitive resistors based on semiconducting oxides. J. Chem. Soc.,. Faraday. Trans. 1995, 91, 4299-307.
43. Lu, H.; Ma, W.; Gao, J.; Li, J. Diffusion-reaction theory for conductance response in metal oxide gas sensing thin films. Sensors. Actuat. B-Chem. 2000, 66, 228-31.
44. Sakai, G.; Matsunaga, N.; Shimanoe, K.; Yamazoe, N. Theory of gas-diffusion controlled sensitivity for thin film semiconductor gas sensor. Sensors. Actuat. B-Chem. 2001, 80, 125-31.
45. Tonezzer, M.; Izidoro, S. C.; Moraes, J. P. A.; Dang, L. T. T. Improved gas selectivity based on carbon modified SnO2 Nanowires. Front. Mater. 2019, 6, 277.
46. Mondal, B.; Gogoi, P. K. Nanoscale heterostructured materials based on metal oxides for a chemiresistive gas sensor. ACS. Appl. Electron. Mater. 2022, 4, 59-86.
47. Li, Y.; Luo, W.; Qin, N.; et al. Highly ordered mesoporous tungsten oxides with a large pore size and crystalline framework for H2S sensing. Angew. Chem. Int. Ed. Engl. 2014, 53, 9035-40.
48. Liu, J.; Huang, H.; Zhao, H.; et al. Enhanced gas sensitivity and selectivity on aperture-controllable 3D interconnected macro-mesoporous ZnO nanostructures. ACS. Appl. Mater. Interfaces. 2016, 8, 8583-90.
49. Meng, L.; Li, Y.; Yang, M.; et al. Temperature-controlled resistive sensing of gaseous H2S or NO2 by using flower-like palladium-doped SnO2 nanomaterials. Mikrochim. Acta. 2020, 187, 297.
50. Motooka, M.; Uno, S. Improvement in limit of detection of enzymatic biogas sensor utilizing chromatography paper for breath analysis. Sensors. (Basel). 2018, 18, 440.
51. Malik, R.; Tomer, V. K.; Mishra, Y. K.; Lin, L. Functional gas sensing nanomaterials: a panoramic view. Appl. Phys. Rev. 2020, 7, 021301.
52. Marikutsa, A.; Rumyantseva, M.; Konstantinova, E. A.; Gaskov, A. The key role of active sites in the development of selective metal oxide sensor materials. Sensors. (Basel). 2021, 21, 2554.
53. Azad, A. M.; Akbar, S. A.; Mhaisalkar, S. G.; Birkefeld, L. D.; Goto, K. S. Solid‐state gas sensors: a review. J. Electrochem. Soc. 2019, 139, 3690-704.
54. Lee, Y.; Kwon, H.; Yoon, J. S.; Kim, J. K. Overcoming ineffective resistance modulation in p-type NiO gas sensor by nanoscale Schottky contacts. Nanotechnology 2019, 30, 115501.
55. Barsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U. Modeling of sensing and transduction for p-type semiconducting metal oxide based gas sensors. J. Electroceram. 2009, 25, 11-9.
56. Li, T.; Cheng, L.; Gao, R.; et al. 2-methylimidazole assisted synthesis of waxberry-like CuO with oxygen vacancies for low-power Cl2 detection. Appl. Surf. Sci. 2024, 655, 159562.
57. Mihalcea, C. G.; Stefan, M.; Ghica, C.; et al. In-depth insight into the structural properties of nanoparticulate NiO for CO sensing. Appl. Surf. Sci. 2024, 651, 159252.
58. Yang, W.; Wang, J.; Zhao, Y.; He, J.; Meng, H. Mesopore engineering of Co3O4 nanoplates for enhanced detection of toluene vapor. Chem. Phys. 2024, 579, 112185.
59. Jung, M. H.; Kwak, M.; Ahn, J.; Song, J. Y.; Kang, H.; Jung, H. T. Highly sensitive and selective acetylene CuO/ZnO heterostructure sensors through electrospinning at lean O2 concentration for transformer diagnosis. ACS. Sens. 2024, 9, 217-27.
60. Liu, D.; Pan, J.; Tang, J.; Liu, W.; Bai, S.; Luo, R. Ag decorated SnO2 nanoparticles to enhance formaldehyde sensing properties. J. Phys. Chem. Solids. 2019, 124, 36-43.
61. Majhi, S. M.; Navale, S. T.; Mirzaei, A.; Kim, H. W.; Kim, S. S. Strategies to boost chemiresistive sensing performance of In2O3-based gas sensors: an overview. Inorg. Chem. Front. 2023, 10, 3428-67.
62. Ma, J.; Xie, W.; Li, J.; et al. Micellar Nanoreactors enabled site-selective decoration of Pt nanoparticles functionalized mesoporous SiO2/WO(3-x) composites for improved CO sensing. Small 2023, 19, e2301011.
63. Chen, K.; Xie, W.; Deng, Y.; et al. Alkaloid precipitant reaction inspired controllable synthesis of mesoporous tungsten oxide spheres for biomarker sensing. ACS. Nano. 2023, 17, 15763-75.
64. Rothschild, A.; Komem, Y. The effect of grain size on the sensitivity of nanocrystalline metal-oxide gas sensors. J. Appl. Phys. 2004, 95, 6374-80.
65. Righettoni, M.; Tricoli, A.; Pratsinis, S. Thermally Stable, Silica-doped ε-WO3 for sensing of acetone in the human breath. Chem. Mater. 2010, 22, 3152-7.
66. Yang, B.; Tran, T.; Milam-guerrero, J.; To, D. T.; Stahovich, T.; Myung, N. V. Enhancing gas sensing performance of tungsten trioxide (WO3) nanofibers through diameter and crystallinity control. Sens. Actuators. Rep. 2024, 7, 100182.
67. Nascimento, E. P.; Firmino, H. C.; Neves, G. A.; Menezes, R. R. A review of recent developments in tin dioxide nanostructured materials for gas sensors. Ceram. Int. 2022, 48, 7405-40.
68. Sun, Y. F.; Liu, S. B.; Meng, F. L.; et al. Metal oxide nanostructures and their gas sensing properties: a review. Sensors. (Basel). 2012, 12, 2610-31.
69. Xu, C.; Tamaki, J.; Miura, N.; Yamazoe, N. Grain size effects on gas sensitivity of porous SnO2-based elements. Sensors. Actuat. B-Chem. 1991, 3, 147-55.
70. Deng, X.; Chen, K.; Tüysüz, H. Protocol for the Nanocasting method: preparation of ordered mesoporous metal oxides. Chem. Mater. 2016, 29, 40-52.
71. Zou, Y.; Zhou, X.; Zhu, Y.; Cheng, X.; Zhao, D.; Deng, Y. sp2-hybridized carbon-containing block copolymer templated synthesis of mesoporous semiconducting metal oxides with excellent gas sensing property. Acc. Chem. Res. 2019, 52, 714-25.
72. Li, X.; Wan, Z.; Hu, Q.; et al. Effect of doping on the morphology and dimethylamine gas sensing properties of hexagonal pine needle-like WO3 structures with highly exposed (100) facet. Appl. Surf. Sci. 2024, 651, 159258.
73. Du, W.; Si, W.; Du, W.; et al. Unraveling the promoted nitrogen dioxide detection performance of N-doped SnO2 microspheres at low temperature. J. Alloys. Compd. 2020, 834, 155209.
74. Ma, J.; Ren, Y.; Zhou, X.; et al. Pt nanoparticles sensitized ordered mesoporous WO3 semiconductor: gas sensing performance and mechanism study. Adv. Funct. Mater. 2017, 28, 1705268.
75. He, J.; Cheng, Z.; Liu, Z.; et al. Epitaxial growth of Co3O4/In2O3 p-n heterostructure with rich oxygen vacancies for ultrahigh triethylamine sensing. Sensors. Actuat. B-Chem. 2024, 400, 134881.
76. Zhao, T.; Qiu, P.; Fan, Y.; et al. Hierarchical branched mesoporous TiO2-SnO2 nanocomposites with well-defined n-n heterojunctions for highly efficient ethanol sensing. Adv. Sci. (Weinh). 2019, 6, 1902008.
77. Alali, K. T.; Liu, J.; Moharram, D.; et al. Fabrication of electrospun Co3O4/CuO p-p heterojunctions nanotubes functionalized with HFIP for detecting chemical nerve agent under visible light irradiation. Sensors. Actuat. B-Chem. 2020, 314, 128076.
78. Zou, Y.; Zhou, X.; Ma, J.; Yang, X.; Deng, Y. Recent advances in amphiphilic block copolymer templated mesoporous metal-based materials: assembly engineering and applications. Chem. Soc. Rev. 2020, 49, 1173-208.
79. Rossinyol, E.; Prim, A.; Pellicer, E.; et al. Synthesis and characterization of chromium-doped mesoporous tungsten oxide for gas sensing applications. Adv. Funct. Mater. 2007, 17, 1801-6.
80. Shi, Y.; Wan, Y.; Liu, R.; Tu, B.; Zhao, D. Synthesis of highly ordered mesoporous crystalline WS2 and MoS2 via a high-temperature reductive sulfuration route. J. Am. Chem. Soc. 2007, 129, 9522-31.
81. Goettmann, F.; Fischer, A.; Antonietti, M.; Thomas, A. Chemical synthesis of mesoporous carbon nitrides using hard templates and their use as a metal-free catalyst for Friedel-Crafts reaction of benzene. Angew. Chem. Int. Ed. Engl. 2006, 45, 4467-71.
82. Shi, Y.; Meng, Y.; Chen, D.; et al. Highly ordered mesoporous silicon carbide ceramics with large surface areas and high stability. Adv. Funct. Mater. 2006, 16, 561-7.
83. Xie, W.; Huang, X. Y.; Zhu, C.; et al. Synthesis of ordered mesoporous metal oxides by solvent evaporation-induced cooperative assembly. Nat. Protoc. 2025.
84. Zhang, Y.; Yang, Q.; Yang, X.; Deng, Y. One-step synthesis of in-situ N-doped ordered mesoporous titania for enhanced gas sensing performance. Microporous. Mesoporous. Mater. 2018, 270, 75-81.
85. Lunkenbein, T.; Kamperman, M.; Li, Z.; et al. Direct synthesis of inverse hexagonally ordered diblock copolymer/polyoxometalate nanocomposite films. J. Am. Chem. Soc. 2012, 134, 12685-92.
86. Eckhardt, B.; Ortel, E.; Bernsmeier, D.; et al. Micelle-templated oxides and carbonates of zinc, cobalt, and aluminum and a generalized strategy for their synthesis. Chem. Mater. 2013, 25, 2749-58.
87. Fan, J.; Boettcher, S. W.; Stucky, G. D. Nanoparticle assembly of ordered multicomponent mesostructured metal oxides via a versatile sol-gel process. Chem. Mater. 2006, 18, 6391-6.
88. Wei, J.; Ren, Y.; Luo, W.; et al. ordered mesoporous alumina with ultra-large pores as an efficient absorbent for selective bioenrichment. Chem. Mater. 2017, 29, 2211-7.
89. Xiao, X.; Liu, L.; Ma, J.; et al. Ordered Mesoporous Tin oxide semiconductors with large pores and crystallized walls for high-performance gas sensing. ACS. Appl. Mater. Interfaces. 2018, 10, 1871-80.
90. Corma, A.; Atienzar, P.; García, H.; Chane-Ching, J. Y. Hierarchically mesostructured doped CeO2 with potential for solar-cell use. Nat. Mater. 2004, 3, 394-7.
91. Buonsanti, R.; Pick, T. E.; Krins, N.; Richardson, T. J.; Helms, B. A.; Milliron, D. J. Assembly of ligand-stripped nanocrystals into precisely controlled mesoporous architectures. Nano. Lett. 2012, 12, 3872-7.
92. Zhou, X.; Ma, J.; Ren, Y.; Zou, Y.; Zhao, D.; Deng, Y. Bridging molecule assisted organic-inorganic interface coassembly to rationally construct metal oxide mesostructures. Chem. Mater. 2022, 34, 6824-34.
93. Rauda, I. E.; Buonsanti, R.; Saldarriaga-Lopez, L. C.; et al. General method for the synthesis of hierarchical nanocrystal-based mesoporous materials. ACS. Nano. 2012, 6, 6386-99.
94. Song, D. P.; Li, C.; Li, W.; Watkins, J. J. Block copolymer nanocomposites with high refractive index contrast for one-step photonics. ACS. Nano. 2016, 10, 1216-23.
95. Yang, X.; Lu, P.; Yu, L.; et al. An efficient emulsion‐induced interface assembly approach for rational synthesis of mesoporous carbon spheres with versatile architectures. Adv. Funct. Mater. 2020, 30, 2002488.
96. Zhang, L.; Cui, T.; Cao, X.; et al. Inorganic-macroion-induced formation of bicontinuous block copolymer nanocomposites with enhanced conductivity and modulus. Angew. Chem. Int. Ed. Engl. 2017, 56, 9013-7.
97. Lee, J.; Orilall, M. C.; Warren, S. C.; Kamperman, M.; DiSalvo, F. J.; Wiesner, U. Direct access to thermally stable and highly crystalline mesoporous transition-metal oxides with uniform pores. Nat. Mater. 2008, 7, 222-8.
98. Zhang, J.; Deng, Y.; Gu, D.; et al. Ligand‐assisted assembly approach to synthesize large‐pore ordered mesoporous titania with thermally stable and crystalline framework. Adv. Energy. Mater. 2011, 1, 241-8.
99. Li, Y.; Zhou, X.; Luo, W.; et al. Pore Engineering of mesoporous tungsten oxides for ultrasensitive gas sensing. Adv. Mater. Inter. 2018, 6, 1801269.
100. Zhou, X.; Zou, Y.; Ma, J.; et al. Cementing mesoporous ZnO with silica for controllable and switchable gas sensing selectivity. Chem. Mater. 2019, 31, 8112-20.
101. Park, K. S.; Ni, Z.; Côté, A. P.; et al. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 10186-91.
102. Shi, L.; Benetti, D.; Wei, Q.; Rosei, F. MOF-Derived In2O3/CuO p-n heterojunction photoanode incorporating graphene nanoribbons for solar hydrogen generation. Small 2023, 19, e2300606.
103. Cavka, J. H.; Jakobsen, S.; Olsbye, U.; et al. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850-1.
104. Kong, L.; Yuan, Z.; Gao, H.; Meng, F. Recent progress of gas sensors based on metal oxide composites derived from bimetallic metal-organic frameworks. TrAC-Trend. Anall. Chem. 2023, 166, 117199.
105. Wang, Y.; Lü, Y.; Zhan, W.; Xie, Z.; Kuang, Q.; Zheng, L. Synthesis of porous Cu2O/CuO cages using Cu-based metal-organic frameworks as templates and their gas-sensing properties. J. Mater. Chem. A. 2015, 3, 12796-803.
106. Jang, J. S.; Koo, W. T.; Kim, D. H.; Kim, I. D. In situ coupling of multidimensional MOFs for heterogeneous metal-oxide architectures: toward sensitive chemiresistors. ACS. Cent. Sci. 2018, 4, 929-37.
107. Li, Y.; Yue, L.; Yue, L.; et al. Metal-organic frameworks-derived hollow nanotube La2O3-In2O3 heterojunctions for enhanced TEA sensing at low temperature. Sensors. Actuat. B-Chem. 2023, 378, 133125.
108. Qin, C.; Wang, B.; Wu, N.; Han, C.; Wang, Y. General strategy to fabricate porous co-based bimetallic metal oxide nanosheets for high-performance CO sensing. ACS. Appl. Mater. Interfaces. 2021, 13, 26318-29.
109. Koo, W. T.; Choi, S. J.; Jang, J. S.; Kim, I. D. Metal-organic framework templated synthesis of ultrasmall catalyst loaded ZnO/ZnCo2O4 hollow spheres for enhanced gas sensing properties. Sci. Rep. 2017, 7, 45074.
110. Okai Amu-Darko, J. N.; Hussain, S.; Zhang, X.; et al. Metal-organic frameworks-derived In2O3/ZnO porous hollow nanocages for highly sensitive H2S gas sensor. Chemosphere 2023, 314, 137670.
111. Ding, Y.; Zhuang, Q.; Guo, X.; et al. NiO-CuO hydrangeas-like composite derived from Ni/Cu bimetallic MOFs for sensitive detection of H2S at room temperature. Appl. Surf. Sci. 2023, 612, 155792.
112. Chen, Q.; Zhang, Y.; Ma, S.; et al. Multishelled NiO/NiCo2O4 hollow microspheres derived from bimetal-organic frameworks as high-performance sensing material for acetone detection. J. Hazard. Mater. 2021, 415, 125662.
113. Koo, W. T.; Choi, S. J.; Kim, S. J.; Jang, J. S.; Tuller, H. L.; Kim, I. D. Heterogeneous sensitization of metal-organic framework driven metal@metal oxide complex catalysts on an oxide nanofiber scaffold toward superior gas sensors. J. Am. Chem. Soc. 2016, 138, 13431-7.
114. Koo, W. T.; Yu, S.; Choi, S. J.; Jang, J. S.; Cheong, J. Y.; Kim, I. D. Nanoscale PdO catalyst functionalized Co3O4 hollow nanocages using MOF templates for selective detection of acetone molecules in exhaled breath. ACS. Appl. Mater. Interfaces. 2017, 9, 8201-10.
115. Feng, Y.; Li, P.; Wei, J. Engineering functional mesoporous materials from plant polyphenol based coordination polymers. Coord. Chem. Rev. 2022, 468, 214649.
116. Wang, G.; Qin, J.; Zhou, X.; et al. Self-template synthesis of mesoporous metal oxide spheres with metal-mediated inner architectures and superior sensing performance. Adv. Funct. Mater. 2018, 28, 1806144.
117. Wang, G.; Zhou, X.; Qin, J.; et al. General synthesis of mixed semiconducting metal oxide hollow spheres with tunable compositions for low-temperature chemiresistive sensing. ACS. Appl. Mater. Interfaces. 2019, 11, 35060-7.
118. Feng, B.; Wu, Y.; Ren, Y.; et al. Self-template synthesis of mesoporous Au-SnO2 nanospheres for low-temperature detection of triethylamine vapor. Sensors. Actuat. B-Chem. 2022, 356, 131358.
119. Wang, G.; Wang, K.; Liu, Z.; et al. Hollow multi-shelled NiO nanoreactor for nanoconfined catalytic degradation of organic pollutants via peroxydisulfate activation. Appl. Catal. B-Environ. 2023, 325, 122359.
120. Chen, Y.; Li, Y.; Feng, B.; Wu, Y.; Zhu, Y.; Wei, J. Self-templated synthesis of mesoporous Au-ZnO nanospheres for seafood freshness detection. Sensors. Actuat. B-Chem. 2022, 360, 131662.
121. Feng, B.; Wang, Z.; Feng, Y.; et al. Single-atom Au-functionalized mesoporous SnO2 nanospheres for ultrasensitive detection of listeria monocytogenes biomarker at low temperatures. ACS. Nano. 2024, 18, 22888-900.
122. Li, P.; Diao, L.; Liao, X.; Wang, Z.; Feng, Y.; Wei, J. Rapid and selective detection of trace hydrogen by mesoporous SnO2 anchored with Au-Pd dual-atom sensitizers. Nano. Lett. 2025, 25, 8243-50.
123. Zhou, J.; Lin, Z.; Ju, Y.; Rahim, M. A.; Richardson, J. J.; Caruso, F. Polyphenol-mediated assembly for particle engineering. Acc. Chem. Res. 2020, 53, 1269-78.
124. Villa, K.; Murcia-lópez, S.; Morante, J. R.; Andreu, T. An insight on the role of La in mesoporous WO3 for the photocatalytic conversion of methane into methanol. Appl. Catal. B-Environ. 2016, 187, 30-6.
125. Wei, D.; Jiang, W.; Gao, H.; et al. Facile synthesis of La-doped In2O3 hollow microspheres and enhanced hydrogen sulfide sensing characteristics. Sensors. Actuat. B-Chem. 2018, 276, 413-20.
126. Kaur, J.; Anand, K.; Kaur, A.; Singh, R. C. Sensitive and selective acetone sensor based on Gd doped WO3/reduced graphene oxide nanocomposite. Sensors. Actuat. B-Chem. 2018, 258, 1022-35.
127. Liu, Y.; Guo, R.; Yuan, K.; et al. Engineering pore walls of mesoporous tungsten oxides via Ce doping for the development of high-performance smart gas sensors. Chem. Mater. 2022, 34, 2321-32.
128. Zhao, Y.; Zou, X.; Chen, H.; Chu, X.; Li, G. Tailoring energy level and surface basicity of metal oxide semiconductors by rare-earth incorporation for high-performance formaldehyde detection. Inorg. Chem. Front. 2019, 6, 1767-74.
129. Bai, J.; Luo, Y.; Chen, C.; et al. Functionalization of 1D In2O3 nanotubes with abundant oxygen vacancies by rare earth dopant for ultra-high sensitive ethanol detection. Sensors. Actuat. B-Chem. 2020, 324, 128755.
130. Wang, L.; Ma, S.; Xu, X.; et al. Oxygen vacancy-based Tb-doped SnO2 nanotubes as an ultra-sensitive sensor for ethanol detection. S. Sensors. Actuat. B-Chem. 2021, 344, 130111.
131. Li, Z.; Wang, W.; Zhao, Z.; Liu, X.; Song, P. One-step hydrothermal preparation of Ce-doped MoO3 nanobelts with enhanced gas sensing properties. RSC. Adv. 2017, 7, 28366-72.
132. Yoon, J. W.; Kim, J. S.; Kim, T. H.; Hong, Y. J.; Kang, Y. C.; Lee, J. H. A New strategy for humidity independent oxide chemiresistors: dynamic self-refreshing of In2O3 sensing surface assisted by layer-by-layer coated CeO2 Nanoclusters. Small 2016, 12, 4229-40.
133. Kwak, C. H.; Kim, T. H.; Jeong, S. Y.; Yoon, J. W.; Kim, J. S.; Lee, J. H. Humidity-independent oxide semiconductor chemiresistors using terbium-doped SnO2 yolk-shell spheres for real-time breath analysis. ACS. Appl. Mater. Interfaces. 2018, 10, 18886-94.
134. Kim, J. S.; Na, C. W.; Kwak, C. H.; et al. Humidity-independent gas sensors Using Pr-Doped In2O3 macroporous spheres: role of Cyclic Pr3+/Pr4+ redox reactions in suppression of water-poisoning effect. ACS. Appl. Mater. Interfaces. 2019, 11, 25322-9.
135. Sukunta, J.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Highly-sensitive H2S sensors based on flame-made V-substituted SnO2 sensing films. Sensors. Actuat. B-Chem. 2017, 242, 1095-107.
136. Sun, J.; Wang, Y.; Song, P.; Yang, Z.; Wang, Q. Metal-organic framework-derived Cr-doped hollow In2O3 nanoboxes with excellent gas-sensing performance toward ammonia. J. Alloys. Compd. 2021, 879, 160472.
137. Li, P.; Cao, C.; Shen, Q.; et al. Cr-doped NiO nanoparticles as selective and stable gas sensor for ppb-level detection of benzyl mercaptan. Sensors. Actuat. B-Chem. 2021, 339, 129886.
138. Qin, C.; Wang, B.; Wang, Y. Metal-organic frameworks-derived Mn-doped Co3O4 porous nanosheets and enhanced CO sensing performance. Sensors. Actuat. B-Chem. 2022, 351, 130943.
139. Wang, D.; Zhai, C.; Du, L.; Gu, K.; Zhang, M. Enhanced triethylamine sensing performance of metal-organic framework derived nest-type Fe-doped NiO nanostructure. Inorg. Chem. Front. 2020, 7, 1474-82.
140. Rong, Q.; Li, Y.; Hao, S.; et al. Raspberry-like mesoporous Co-doped TiO2 nanospheres for a high-performance formaldehyde gas sensor. J. Mater. Chem. A. 2021, 9, 6529-37.
141. Chen, K.; Zhou, Y.; Jin, R.; et al. Gas sensor based on cobalt-doped 3D inverse opal SnO2 for air quality monitoring. Sensors. Actuat. B-Chem. 2022, 350, 130807.
142. Jin, Z.; Wang, C.; Wu, L.; et al. Fast responding and recovering of NO2 sensors based on Ni-doped In2O3 nanoparticles. Sensors. Actuat. B-Chem. 2023, 377, 133058.
143. Li, J.; Zheng, M.; Yang, M.; et al. Three-in-one Ni doped porous SnO2 nanorods sensor: controllable oxygen vacancies content, surface site activation and low power consumption for highly selective NO2 monitoring. Sensors. Actuat. B-Chem. 2023, 382, 133550.
144. Zhang, S.; Zhang, B.; Li, W.; et al. Electrospun copper-doped tungsten oxide nanowires for triethylamine gas sensing. Vacuum 2023, 215, 112377.
145. Wang, L.; Ma, S.; Li, J.; et al. Mo-doped SnO2 nanotubes sensor with abundant oxygen vacancies for ethanol detection. Sensors. Actuat. B-Chem. 2021, 347, 130642.
146. Du, L.; Sun, H.; Liu, Y. Metal-organic framework-derived hierarchical flower-like Mo-doped Co3O4 for enhanced triethylamine sensing properties. J. Alloys. Compd. 2022, 900, 163470.
147. Raghu, A. V.; Karuppanan, K. K.; Pullithadathil, B. Controlled carbon doping in anatase TiO2(101) facets: superior trace-level ethanol gas sensor performance and adsorption kinetics. Adv. Mater. Inter. 2019, 6, 1801714.
148. Zhao, D.; Zhang, X.; Sui, L.; et al. C-doped TiO2 nanoparticles to detect alcohols with different carbon chains and their sensing mechanism analysis. Sensors. Actuat. B-Chem. 2020, 312, 127942.
149. Wang, M.; Li, Y.; Yao, B.; Zhai, K.; Li, Z.; Yao, H. Synthesis of three-dimensionally ordered macro/mesoporous C-doped WO3 materials: Effect of template sizes on gas sensing properties. Sensors. Actuat. B-Chem. 2019, 288, 656-66.
150. Mo, X.; Zhu, C.; Zhang, Z.; et al. Nitrogen-doped indium oxide electrochemical sensor for stable and selective NO2 detection. Adv. Mater. 2024, 36, e2409294.
151. Abbasi, A.; Jahanbin Sardroodi, J. N-doped TiO2 anatase nanoparticles as a highly sensitive gas sensor for NO2 detection: insights from DFT computations. Environ. Sci:. Nano. 2016, 3, 1153-64.
152. Wang, K.; Peng, T.; Wang, Z.; et al. Correlation between the H2 response and its oxidation over TiO2 and N doped TiO2 under UV irradiation induced by Fermi level. Appl. Catal. B-Environ. 2019, 250, 89-98.
153. Ren, Y.; Zou, Y.; Liu, Y.; et al. Synthesis of orthogonally assembled 3D cross-stacked metal oxide semiconducting nanowires. Nat. Mater. 2020, 19, 203-11.
154. Ren, Y.; Xie, W.; Li, Y.; et al. Dynamic coassembly of amphiphilic block copolymer and polyoxometalates in dual solvent systems: an efficient approach to heteroatom-doped semiconductor metal oxides with controllable nanostructures. ACS. Cent. Sci. 2022, 8, 1196-208.
155. Güntner, A. T.; Righettoni, M.; Pratsinis, S. E. Selective sensing of NH3 by Si-doped α-MoO3 for breath analysis. Sensors. Actuat. B-Chem. 2016, 223, 266-73.
156. Shuvo, S. N.; Ulloa Gomez, A. M.; Mishra, A.; Chen, W. Y.; Dongare, A. M.; Stanciu, L. A. Sulfur-doped titanium carbide MXenes for room-temperature gas sensing. ACS. Sens. 2020, 5, 2915-24.
157. Li, W.; He, L.; Bai, X.; et al. Enhanced NO2 sensing performance of S-doped biomorphic SnO2 with increased active sites and charge transfer at room temperature. Inorg. Chem. Front. 2020, 7, 2031-42.
158. Xu, K.; Tian, S.; Zhu, J.; et al. High selectivity of sulfur-doped SnO2 in NO2 detection at lower operating temperatures. Nanoscale 2018, 10, 20761-71.
159. Deng, Y.; Chen, K.; Xie, W.; et al. On-chip construction of hierarchically macro-/mesoporous cerium oxide/Pt gas sensitive film for ultrasensitive detection of trace oxygen. Interdiscip. Mater. 2025, 4, 585-98.
160. Li, J.; Xue, L.; Deng, Y.; et al. A regiospecific co-assembly method to functionalize ordered mesoporous metal oxides with customizable noble metal nanocrystals. ACS. Cent. Sci. 2024, 10, 2274-84.
161. Ren, Y.; Xie, W.; Li, Y.; et al. Noble metal nanoparticles decorated metal oxide semiconducting nanowire arrays interwoven into 3D mesoporous superstructures for low-temperature gas sensing. ACS. Cent. Sci. 2021, 7, 1885-97.
162. Wang, Y.; Liu, J.; Cui, X.; et al. NH3 gas sensing performance enhanced by Pt-loaded on mesoporous WO3. Sensors. Actuat. B-Chem. 2017, 238, 473-81.
163. Liu, B.; Zhang, L.; Luo, Y.; Gao, L.; Duan, G. The dehydrogenation of H-S Bond into sulfur species on supported Pd single atoms allows highly selective and sensitive hydrogen sulfide detection. Small 2021, 17, e2105643.
164. Kim, S. J.; Choi, S. J.; Jang, J. S.; et al. Mesoporous WO3 nanofibers with protein-templated nanoscale catalysts for detection of trace biomarkers in exhaled breath. ACS. Nano. 2016, 10, 5891-9.
165. Park, S.; Lim, Y.; Oh, D.; et al. Steering selectivity in the detection of exhaled biomarkers over oxide nanofibers dispersed with noble metals. J. Mater. Chem. A. 2023, 11, 3535-45.
166. Sui, N.; Wei, X.; Cao, S.; Zhang, P.; Zhou, T.; Zhang, T. Nanoscale bimetallic AuPt-functionalized metal oxide chemiresistors: Ppb-level and selective detection for ozone and acetone. ACS. Sens. 2022, 7, 2178-87.
167. Miller, D. R.; Akbar, S. A.; Morris, P. A. Nanoscale metal oxide-based heterojunctions for gas sensing: a review. Sensors. Actuat. B-Chem. 2014, 204, 250-72.
168. Wang, C.; Du, L.; Xing, X.; et al. Radial ZnO nanorods decorating Co3O4 nanoparticles for highly selective and sensitive detection of the 3-hydroxy-2-butanone biomarker. Nanoscale 2022, 14, 482-91.
169. Xie, J.; Liu, X.; Jing, S.; Pang, C.; Liu, Q.; Zhang, J. Chemical and electronic modulation via atomic layer deposition of NiO on porous In2O3 films to boost NO2 detection. ACS. Appl. Mater. Interfaces. 2021, 13, 39621-32.
170. Xiao, X.; Zhou, X.; Ma, J.; et al. Rational synthesis and gas sensing performance of ordered mesoporous semiconducting WO3/NiO Composites. ACS. Appl. Mater. Interfaces. 2019, 11, 26268-76.
171. Shao, F.; Hoffmann, M.; Prades, J.; et al. Heterostructured p-CuO (nanoparticle)/n-SnO2 (nanowire) devices for selective H2S detection. Sensors. Actuat. B-Chem. 2013, 181, 130-5.
172. Jeong, H. M.; Kim, J. H.; Jeong, S. Y.; Kwak, C. H.; Lee, J. H. Co3O4-SnO2 hollow heteronanostructures: facile control of gas selectivity by compositional tuning of sensing materials via galvanic replacement. ACS. Appl. Mater. Interfaces. 2016, 8, 7877-83.
173. Woo, H. S.; Na, C. W.; Kim, I. D.; Lee, J. H. Highly sensitive and selective trimethylamine sensor using one-dimensional ZnO-Cr2O3 hetero-nanostructures. Nanotechnology 2012, 23, 245501.
174. Han, J.; Wang, T. Y.; Li, T. T.; Yu, H.; Yang, Y.; Dong, X. T. Enhanced NO gas sensing properties of ordered mesoporous WO3/ZnO prepared by electroless plating. Adv. Materials. Inter. 2017, 5, 1701167.
175. Bhuvaneshwari, S.; Gopalakrishnan, N. Effect of Fe doping on the NH3 sensing properties of CuO nanostructures. J. Mater. Sci:. Mater. Electron. 2019, 30, 6920-8.
176. Li, Z.; Li, H.; Wu, Z.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470-506.
177. Cao, Y.; He, Y.; Zou, X.; Li, G. Tungsten oxide clusters decorated ultrathin In2O3 nanosheets for selective detecting formaldehyde. Sensors. Actuat. B-Chem. 2017, 252, 232-8.
178. Wang, C.; Li, Y.; Qiu, P.; et al. Controllable synthesis of highly crystallized mesoporous TiO2/WO3 heterojunctions for acetone gas sensing. Chin. Chem. Lett. 2020, 31, 1119-23.
179. Zhang, H.; Guo, S.; Zheng, W.; et al. Facile engineering of metal-organic framework derived SnO2-ZnO composite based gas sensor toward superior acetone sensing performance. Chem. Eng. J. 2023, 469, 143927.
180. Gao, X.; Ouyang, Q.; Zhu, C.; Zhang, X.; Chen, Y. Porous MoO3/SnO2 nanoflakes with n-n junctions for sensing H2S. ACS. Appl. Nano. Mater. 2019, 2, 2418-25.
181. Wan, K.; Wang, D.; Wang, F.; et al. Hierarchical In2O3@SnO2 Core-shell nanofiber for high efficiency formaldehyde detection. ACS. Appl. Mater. Interfaces. 2019, 11, 45214-25.
182. Wang, T.; Zhang, S.; Yu, Q.; et al. 3D inverse opal nanostructured multilayer films of two-component heterostructure composites: a new-generation synthetic route and potential application as high-performance acetone detector. Sensors. Actuat. B-Chem. 2018, 276, 262-70.
183. Suh, J. M.; Sohn, W.; Shim, Y. S.; et al. p-p heterojunction of nickel oxide-decorated cobalt oxide nanorods for enhanced sensitivity and selectivity toward volatile organic compounds. ACS. Appl. Mater. Interfaces. 2018, 10, 1050-8.
184. Xu, H.; Zhang, J.; Rehman, A. U.; et al. Synthesis of NiO@CuO nanocomposite as high-performance gas sensing material for NO2 at room temperature. Appl. Surf. Sci. 2017, 412, 230-7.
185. Hozák, P.; Vorokhta, M.; Khalakhan, I.; et al. New insight into the gas-sensing properties of CuOx nanowires by near-ambient pressure XPS. J. Phys. Chem. C. 2019, 123, 29739-49.
186. Gao, H.; Guo, J.; Li, Y.; et al. Highly selective and sensitive xylene gas sensor fabricated from NiO/NiCr2O4 p-p nanoparticles. Sensors. Actuat. B-Chem. 2019, 284, 305-15.
187. Zhang, S.; Li, Y.; Sun, G.; et al. Synthesis of NiO-decorated ZnO porous nanosheets with improved CH4 sensing performance. Appl. Surf. Sci. 2019, 497, 143811.
188. Li, X.; Tan, T.; Ji, W.; et al. Remarkably enhanced methane sensing performance at room temperature via constructing a self-assembled mulberry‐Like ZnO/SnO2 hierarchical structure. Energy. &. Environ. Mater. 2023, 7, e12624.
189. Yao, L.; Li, Y.; Ran, Y.; et al. Construction of novel Pd-SnO2 composite nanoporous structure as a high-response sensor for methane gas. J. Alloys. Compd. 2020, 826, 154063.
190. Li, X.; He, H.; Tan, T.; et al. Annealing effect on the methane sensing performance of Pt-SnO2/ZnO double layer sensor. Appl. Surf. Sci. 2023, 640, 158428.
191. Katoch, A.; Kim, J. H.; Kwon, Y. J.; Kim, H. W.; Kim, S. S. Bifunctional sensing mechanism of SnO2-ZnO composite nanofibers for drastically enhancing the sensing behavior in H2 gas. ACS. Appl. Mater. Interfaces. 2015, 7, 11351-8.
192. Zhang, H.; Wei, W.; Tao, T.; et al. Hierarchical NiO/TiO2 heterojuntion-based conductometric hydrogen sensor with anti-CO-interference. Sensors. Actuat. B-Chem. 2023, 380, 133321.
193. Kim, J.; Mirzaei, A.; Kim, H. W.; Kim, S. S. Improving the hydrogen sensing properties of SnO2 nanowire-based conductometric sensors by Pd-decoration. Sensors. Actuat. B-Chem. 2019, 285, 358-67.
194. Meng, X.; Bi, M.; Gao, W. PdAg alloy modified SnO2 nanoparticles for ultrafast detection of hydrogen. Sensors. Actuat. B-Chem. 2023, 382, 133515.
195. Dhage, S. B.; Patil, V. L.; Patil, P. S.; Ryu, J.; Patil, D. R.; Malghe, Y. S. Synthesis and characterization of CuO-SnO2 nanocomposite for CO gas sensing application. Mater. Lett. 2021, 305, 130831.
196. Zhang, Y.; Wang, Y.; Zhu, L.; Zhang, R.; Cao, J. Enhanced CO sensing performance of WO3 nanorods with PtAg nanoparticles modification: a combined experimental and first-principle study. Vacuum 2021, 193, 110526.
197. Ma, J.; Li, Y.; Zhou, X.; et al. Au nanoparticles decorated mesoporous SiO2-WO3 hybrid materials with improved pore connectivity for ultratrace ethanol detection at low operating temperature. Small 2020, 16, e2004772.
198. Lei, M.; Gao, M.; Yang, X.; et al. Size-controlled Au nanoparticles incorporating mesoporous ZnO for sensitive ethanol sensing. ACS. Appl. Mater. Interfaces. 2021, 13, 51933-44.
199. Song, Z.; Tang, W.; Chen, Z.; et al. Temperature-modulated selective detection of part-per-trillion NO2 using platinum nanocluster sensitized 3D metal oxide nanotube arrays. Small 2022, 18, e2203212.
200. Cao, P.; Cai, Y.; Pawar, D.; et al. Au@ZnO/rGO nanocomposite-based ultra-low detection limit highly sensitive and selective NO2 gas sensor. J. Mater. Chem. C. 2022, 10, 4295-305.
201. Park, S.; Jeon, S.; Kim, H.; et al. Imparting metal oxides with high sensitivity toward light-activated NO2 detection via tailored interfacial chemistry. Adv. Funct. Mater. 2023, 33, 2214008.
202. Li, X. Y.; Sun, G. T.; Fan, F.; et al. Au25 nanoclusters incorporating three-dimensionally ordered macroporous In2O3 for highly sensitive and selective formaldehyde sensing. ACS. Appl. Mater. Interfaces. 2022, 14, 564-73.
203. Hu, L.; Jia, F.; Wang, S.; et al. The nano-composite of Co-doped g-C3N4 and ZnO sensors for the rapid detection of BTEX gases: stability studies and gas sensing mechanism. J. Mater. Sci. 2020, 56, 5041-52.
204. Cao, Z.; Ge, Y.; Wang, W.; et al. Chemical discrimination of benzene series and molecular recognition of the sensing process over Ti-Doped Co3O4. ACS. Sens. 2022, 7, 1757-65.
205. Moon, Y. K.; Jeong, S. Y.; Jo, Y. M.; Jo, Y. K.; Kang, Y. C.; Lee, J. H. Highly Selective detection of benzene and discrimination of volatile aromatic compounds using oxide chemiresistors with tunable Rh-TiO2 catalytic overlayers. Adv. Sci. (Weinh). 2021, 8, 2004078.
206. Wang, D.; Yin, Y.; Xu, P.; et al. The catalytic-induced sensing effect of triangular CeO2 nanoflakes for enhanced BTEX vapor detection with conventional ZnO gas sensors. J. Mater. Chem. A. 2020, 8, 11188-94.
207. Zhou, S.; Lu, Q.; Chen, M.; et al. Platinum-supported cerium-doped indium oxide for highly sensitive triethylamine gas sensing with good antihumidity. ACS. Appl. Mater. Interfaces. 2020, 12, 42962-70.
208. Zeng, J.; Rong, Q.; Xiao, B.; et al. Single-atom silver loaded on tungsten oxide with oxygen vacancies for high performance triethylamine gas sensors. J. Mater. Chem. A. 2021, 9, 8704-10.
209. Ma, J.; Li, Y.; Li, J.; et al. Rationally designed dual-mesoporous transition metal oxides/noble metal nanocomposites for fabrication of gas sensors in real-time detection of 3-hydroxy-2-butanone biomarker. Adv. Funct. Mater. 2021, 32, 2107439.
210. Shao, S.; Yan, L.; Zhang, L.; et al. Data-driven exploration of terbium-doped tungsten oxide for ultra-precise detection of 3H-2B: implications for gas sensor applications. Chem. Eng. J. 2024, 487, 149680.
211. Yang, X.; Shi, Y.; Xie, K.; Fang, S.; Zhang, Y.; Deng, Y. Cocrystallization enabled spatial self-confinement approach to synthesize crystalline porous metal oxide nanosheets for gas sensing. Angew. Chem. Int. Ed. Engl. 2022, 61, e202207816.
212. Wang, D.; Deng, L.; Cai, H.; et al. Bimetallic PtCu nanocrystal sensitization WO3 hollow spheres for highly efficient 3-hydroxy-2-butanone biomarker detection. ACS. Appl. Mater. Interfaces. 2020, 12, 18904-12.
213. Xie, S.; Zhao, C.; Shen, J.; et al. Hierarchical flower-like WO3 nanospheres decorated with bimetallic au and pd for highly sensitive and selective detection of 3-hydroxy-2-butanone biomarker. ACS. Sens. 2023, 8, 728-38.
214. Yang, T.; Zhang, X.; Shiu, B.; Lou, C.; Lin, J.; Li, T. Wearable smart yarn sensor based on ZnO/SnO2 heterojunction for ammonia detecting. J. Mater. Sci. 2022, 57, 21946-59.
215. Ajjaq, A.; Bulut, F.; Ozturk, O.; Acar, S. Advanced NH3 detection by 1D nanostructured La:ZnO sensors with novel intrinsic p-n shifting and ultrahigh baseline stability. ACS. Sens. 2024, 9, 895-911.
216. Wang, Y.; Zhang, S.; Huang, C.; et al. Mesoporous WO3 modified by Au nanoparticles for enhanced trimethylamine gas sensing properties. Dalton. Trans. 2021, 50, 970-8.
217. Shen, J.; Xu, S.; Zhao, C.; et al. Bimetallic Au@Pt nanocrystal sensitization mesoporous α-Fe2O3 hollow nanocubes for highly sensitive and rapid detection of fish freshness at low temperature. ACS. Appl. Mater. Interfaces. 2021, 13, 57597-608.
218. Kim, J.; Kim, K. B.; Li, H.; et al. Pure and Pr-doped Ce4W9O33 with superior hydroxyl scavenging ability: humidity-independent oxide chemiresistors. J. Mater. Chem. A. 2021, 9, 16359-69.
219. Meng, D.; Qiao, T.; Wang, G.; et al. NiO-functionalized In2O3 flower-like structures with enhanced trimethylamine gas sensing performance. Appl. Surf. Sci. 2022, 577, 151877.
220. Yuan, K.; Wang, C. Y.; Zhu, L. Y.; et al. Fabrication of a micro-electromechanical system-based acetone gas sensor using CeO2 nanodot-decorated WO3 nanowires. ACS. Appl. Mater. Interfaces. 2020, 12, 14095-104.
221. Verma, A.; Yadav, B. C. Development and integration of a hierarchical Pd/WO3 acetone-sensing device for real-time exhaled breath monitoring with disposable face mask. J. Hazard. Mater. 2024, 463, 132872.
222. Jeong, S. Y.; Moon, Y. K.; Kim, J. K.; et al. A General solution to mitigate water poisoning of oxide chemiresistors: bilayer sensors with Tb4O7 overlayer. Adv. Funct. Mater. 2020, 31, 2007895.
223. Lei, M.; Zhou, X.; Zou, Y.; et al. A facile construction of heterostructured ZnO/Co3O4 mesoporous spheres and superior acetone sensing performance. Chin. Chem. Lett. 2021, 32, 1998-2004.
224. Shin, H.; Ko, J.; Park, C.; et al. Sacrificial template-assisted synthesis of inorganic nanosheets with high-loading single-atom catalysts: a general approach. Adv. Funct. Mater. 2021, 32, 2110485.
225. Huang, W.; Ding, Q.; Wang, H.; et al. Design of stretchable and self-powered sensing device for portable and remote trace biomarkers detection. Nat. Commun. 2023, 14, 5221.
226. Shatalin, K.; Nuthanakanti, A.; Kaushik, A.; et al. Inhibitors of bacterial H2S biogenesis targeting antibiotic resistance and tolerance. Science 2021, 372, 1169-75.
227. Yan, J.; Guo, X.; Zhu, Y.; Song, Z.; Lee, L. Y. S. Solution-processed metal doping of sub-3 nm SnO2 quantum wires for enhanced H2S sensing at low temperature. J. Mater. Chem. A. 2022, 10, 15657-64.
228. Choi, S.; Ku, K. H.; Kim, B. J.; Kim, I. Novel templating route using Pt infiltrated block copolymer microparticles for catalytic Pt functionalized macroporous WO3 nanofibers and its application in breath pattern recognition. ACS. Sens. 2016, 1, 1124-31.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data






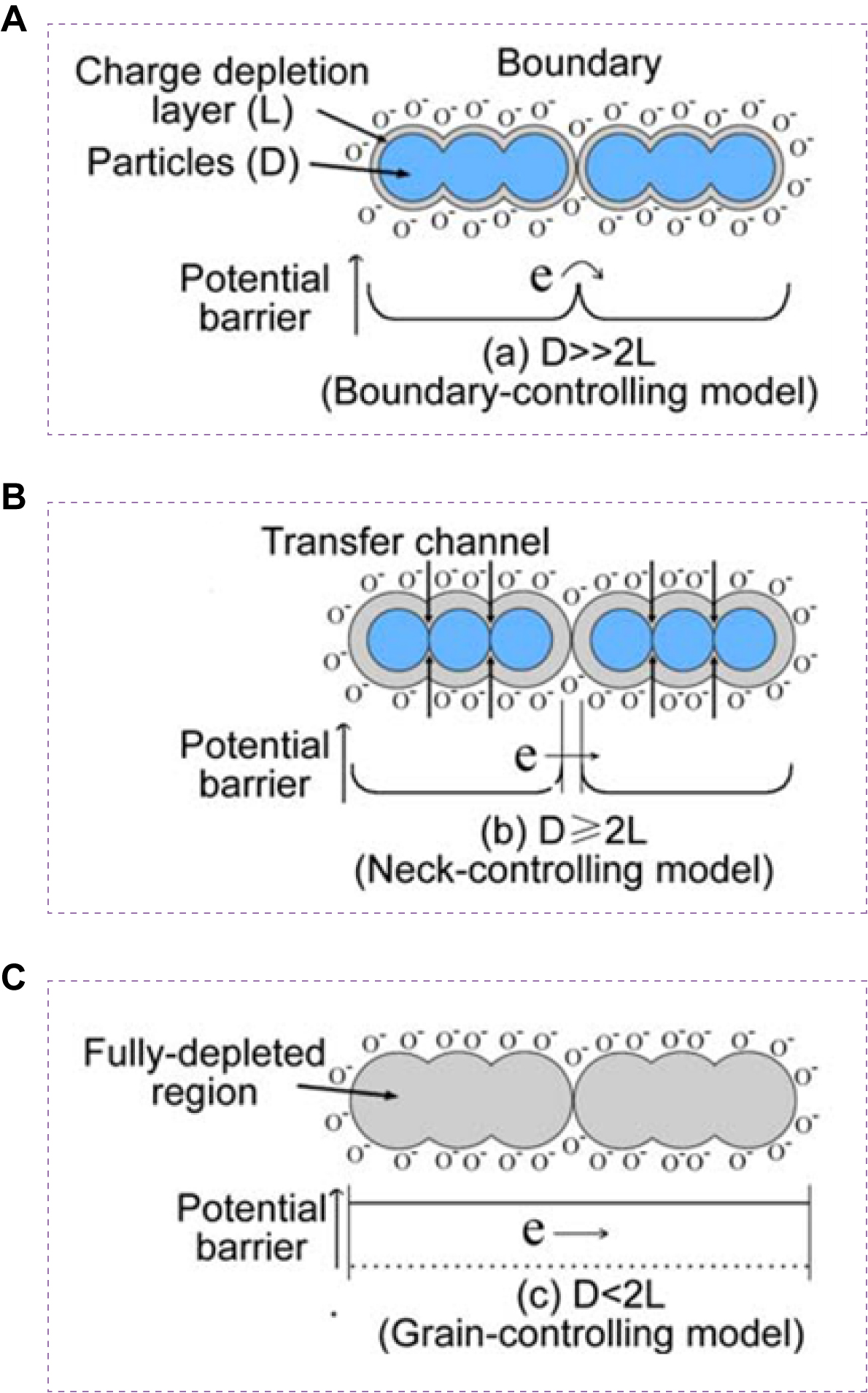
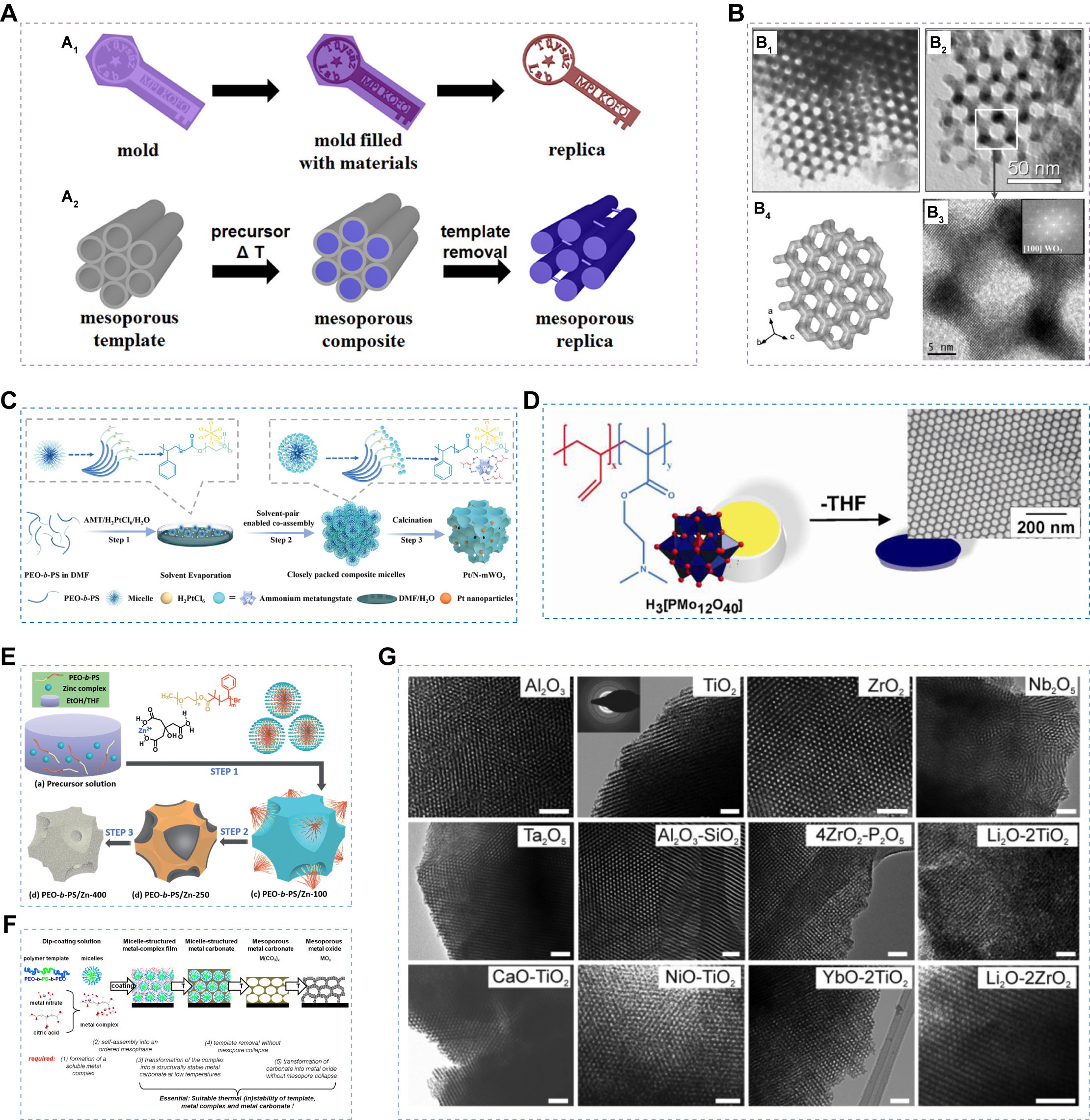
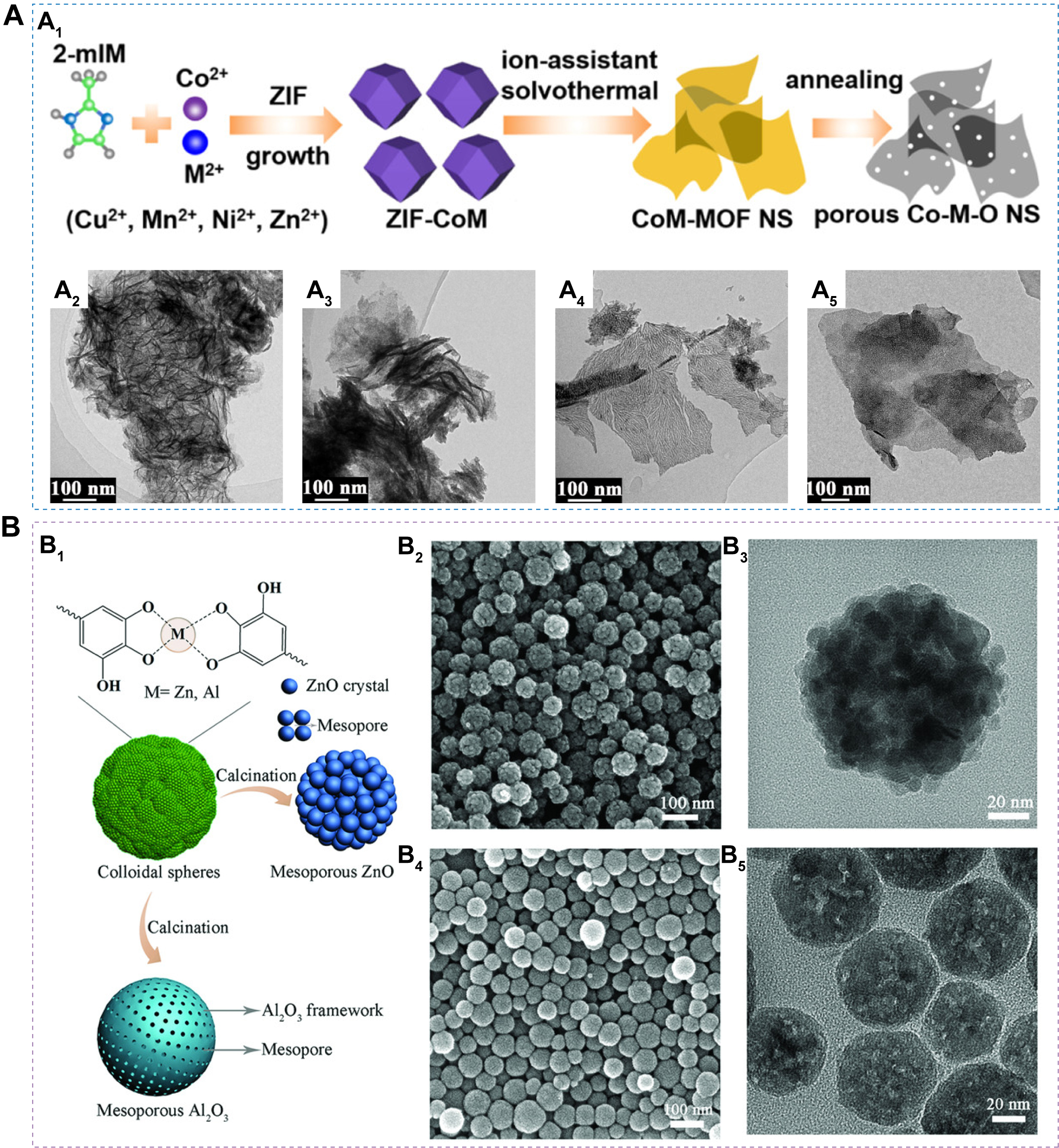
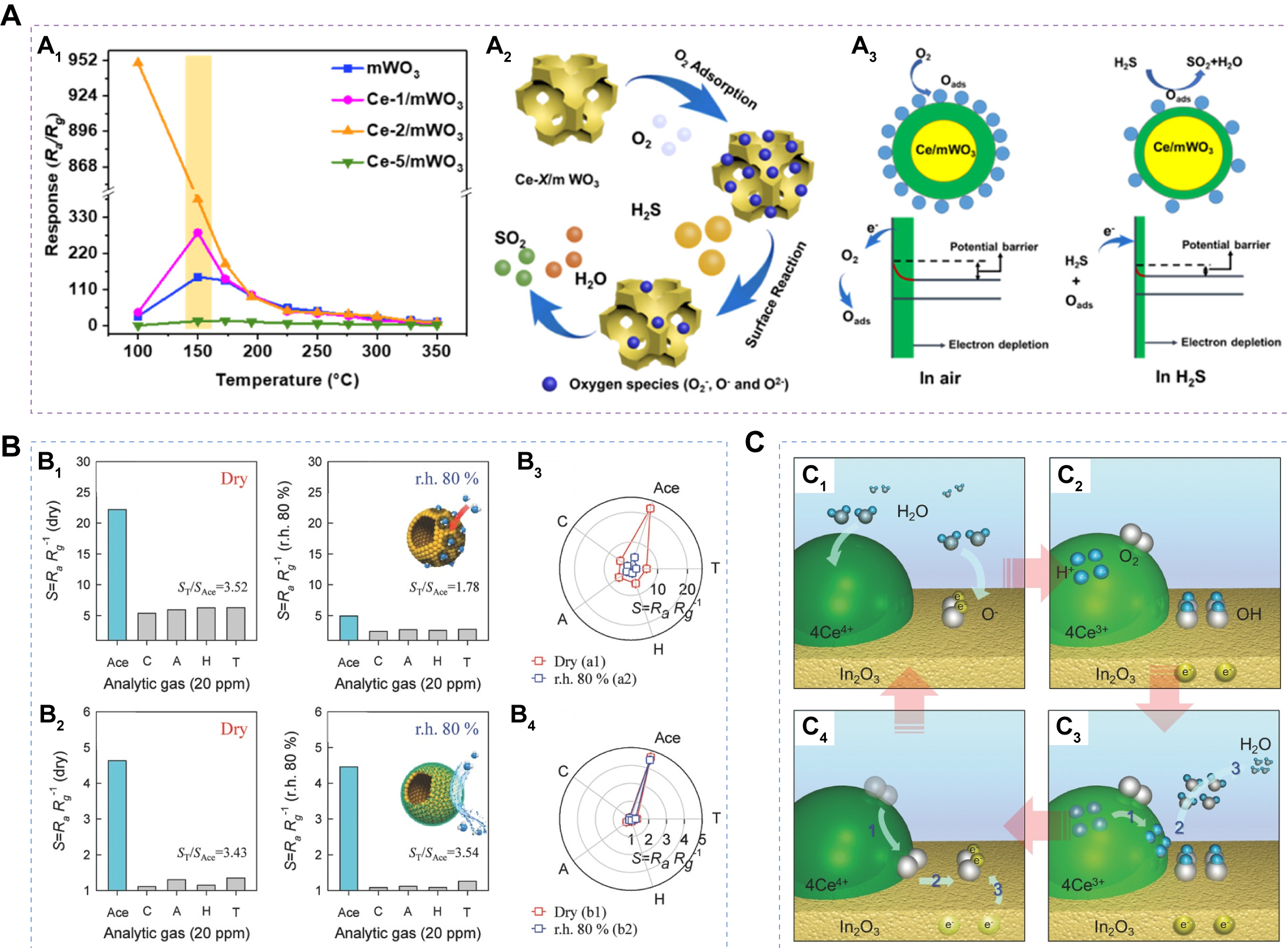









Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].